So, look at the above diagram and see how many valence electrons we used till now and how many are left. Now its time to use another alternative method to determine the molecular geometry of H2O2 that is the AXN method. Oxygen atoms should hold negative charges because electronegativity of oxygen atom is higher than Definitions of molecular mass, molecular weight, molar mass and molar weight. So, the total number of lone pairs present on the central atom of the H2O2 lewis structure is 2 + 2 = 4. The single bond, double bond, or even triple bond around the atom will be counted as one region. View all posts by Priyanka . There are -2 charge on C2O42- ion. Total valence These valence electrons are represented by drawing dots around the individual atoms, hence the Lewis dot structure. (have -1 negative charges) and other carbon atom. As a result, Hydrogen peroxide or H2O2 has a tetrahedral geometry. (Look at the 4th step structure). 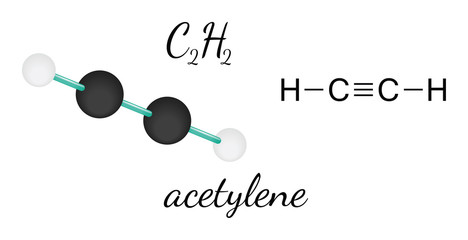 [1] It can also be thought of as the dehydrated form of glyoxylic acid (H(C=O)COOH), or a ketone of ethenone H2C=C=O. Well, that rhymed. The molecular geometry of CO2 is Linear. Ask your chemistry questions and find the answers, Sandmeyer reactions of benzenediazonium chloride, Center atom selection according to the maximum valence.
[1] It can also be thought of as the dehydrated form of glyoxylic acid (H(C=O)COOH), or a ketone of ethenone H2C=C=O. Well, that rhymed. The molecular geometry of CO2 is Linear. Ask your chemistry questions and find the answers, Sandmeyer reactions of benzenediazonium chloride, Center atom selection according to the maximum valence.  Monoisotopic mass 55.989830 Da. = Number of atoms attached to the central atom. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. { Polarity is a chemical property of elements through which they develop poles separating negative and positive charges. How to tell if a molecule is polar or nonpolar? Two lone pairs on each oxygen atom. From my understanding, SO2 also has a double bond between the sulfur atom and the oxygen atoms, so would it also have a linear geometry? Hence, each oxygen needs only 4 valence electrons around them for completing their octets. In CO2, the carbon (C) central atom has no lone pair and is attached to two oxygen (O) atoms. Molecular Geometry of Acetylene (C2H2)# Studying the molecule geometry of a molecule is a fundamental step in chemistry to analyze the behavioral properties of any molecule. The bond angle for H2O2 in its gas phase is 94.8and has a bond angle of 101.9. So, for now, place Carbon in the center position and draw four dots around it. N represents the lone pair of electrons on the central atom, According to the VSEPR chart, if any molecule has the AX. XeF4 Lewis structure, Molecular geometry, Bond angle, Shape, SO2 Lewis structure, Molecular geometry, Bond angle, Shape, SO3 Lewis structure, Molecular geometry, Bond angle, Shape, H2O Lewis structure, Molecular geometry, Bond angle, Shape, O3 Lewis structure, Molecular geometry, Bond angle, Shape, XeF2 Lewis structure, Molecular geometry, Bond angle, Shape, HCN Lewis structure, Molecular geometry, Bond angle, Shape, H2S Molecular geometry, SH2 Lewis structure, Bond angle,, AX3E2 Molecular shape, Bond angle, Hybridization, Polarity, AX4 Molecular shape, Bond angle, Hybridization, Polarity. Here, we use the concept of valence electrons to find out the type of bond formation. The bond angle of CO2 is 180. This molecule can be a good start for beginners who want to learn the fundamentals of such concepts and want to know how to draw Lewis dot structures for other molecules as well. [ 2 dot electrons means 1 lone pair]. Step 3: Find the central atom to begin drawing the structure: As both the elements (carbon and hydrogen) are participating in equal numbers, there will be no central atom. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other. For four oxygen atoms, twelve electrons pairs are spent. The formal charge shows that which atom has more positive or negative charge present on it.
Monoisotopic mass 55.989830 Da. = Number of atoms attached to the central atom. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. { Polarity is a chemical property of elements through which they develop poles separating negative and positive charges. How to tell if a molecule is polar or nonpolar? Two lone pairs on each oxygen atom. From my understanding, SO2 also has a double bond between the sulfur atom and the oxygen atoms, so would it also have a linear geometry? Hence, each oxygen needs only 4 valence electrons around them for completing their octets. In CO2, the carbon (C) central atom has no lone pair and is attached to two oxygen (O) atoms. Molecular Geometry of Acetylene (C2H2)# Studying the molecule geometry of a molecule is a fundamental step in chemistry to analyze the behavioral properties of any molecule. The bond angle for H2O2 in its gas phase is 94.8and has a bond angle of 101.9. So, for now, place Carbon in the center position and draw four dots around it. N represents the lone pair of electrons on the central atom, According to the VSEPR chart, if any molecule has the AX. XeF4 Lewis structure, Molecular geometry, Bond angle, Shape, SO2 Lewis structure, Molecular geometry, Bond angle, Shape, SO3 Lewis structure, Molecular geometry, Bond angle, Shape, H2O Lewis structure, Molecular geometry, Bond angle, Shape, O3 Lewis structure, Molecular geometry, Bond angle, Shape, XeF2 Lewis structure, Molecular geometry, Bond angle, Shape, HCN Lewis structure, Molecular geometry, Bond angle, Shape, H2S Molecular geometry, SH2 Lewis structure, Bond angle,, AX3E2 Molecular shape, Bond angle, Hybridization, Polarity, AX4 Molecular shape, Bond angle, Hybridization, Polarity. Here, we use the concept of valence electrons to find out the type of bond formation. The bond angle of CO2 is 180. This molecule can be a good start for beginners who want to learn the fundamentals of such concepts and want to know how to draw Lewis dot structures for other molecules as well. [ 2 dot electrons means 1 lone pair]. Step 3: Find the central atom to begin drawing the structure: As both the elements (carbon and hydrogen) are participating in equal numbers, there will be no central atom. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other. For four oxygen atoms, twelve electrons pairs are spent. The formal charge shows that which atom has more positive or negative charge present on it.  Or are there differences in the electron configurations that cause the two compounds to have different geometries? stretch the structure making it look like an open book structure. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. Now, as two oxygen atoms need two electrons each to complete their octets, it will share two electrons from the Carbon atom and form double bonds. Atoms that show sp hybridization always have a linear molecular geometry where two sp orbitals will be held at 180 to each other. What is the molecular geometry of H2O2 and its Hybridization? Now, there are three It has a linear geometry arrangement like O=C=O. For H2O2, the notation is AX2N2 which corresponds to tetrahedral geometry in the table given below. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. Thank you very much. This hybridization leads to the formation of new 4 sp hybridized orbitals where the carbon-hydrogen bonding will produce 2 new sp hybridized orbitals. Now look at the above H2O2 lewis dot structure, each oxygen has 4 unshared electrons(2 lone pairs) and 4 shared electrons. It is used as fuel cells and also as a component of the mobile phase of reverse-phase high-performance liquid chromatography methods. Three factors that indicate the polarity of H2O2. Why the H2O2 lewis structure molecular geometry is bent and electron geometry is tetrahedral? These two hybridized orbitals overlap with the two p-orbitals of the Oxygen atom that results in the formation of sigma bonds. So, we will have carbon as the central atom in formic acid. So, these charges create two dipole moments around OH bond in the H2O2 lewis structure which will not cancel out because they are lying in two different planes. Web0. By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons.
Or are there differences in the electron configurations that cause the two compounds to have different geometries? stretch the structure making it look like an open book structure. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. Now, as two oxygen atoms need two electrons each to complete their octets, it will share two electrons from the Carbon atom and form double bonds. Atoms that show sp hybridization always have a linear molecular geometry where two sp orbitals will be held at 180 to each other. What is the molecular geometry of H2O2 and its Hybridization? Now, there are three It has a linear geometry arrangement like O=C=O. For H2O2, the notation is AX2N2 which corresponds to tetrahedral geometry in the table given below. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. Thank you very much. This hybridization leads to the formation of new 4 sp hybridized orbitals where the carbon-hydrogen bonding will produce 2 new sp hybridized orbitals. Now look at the above H2O2 lewis dot structure, each oxygen has 4 unshared electrons(2 lone pairs) and 4 shared electrons. It is used as fuel cells and also as a component of the mobile phase of reverse-phase high-performance liquid chromatography methods. Three factors that indicate the polarity of H2O2. Why the H2O2 lewis structure molecular geometry is bent and electron geometry is tetrahedral? These two hybridized orbitals overlap with the two p-orbitals of the Oxygen atom that results in the formation of sigma bonds. So, we will have carbon as the central atom in formic acid. So, these charges create two dipole moments around OH bond in the H2O2 lewis structure which will not cancel out because they are lying in two different planes. Web0. By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons. 
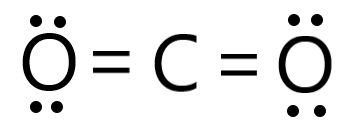 To summarize this blog, we can say that Carbon Dioxide has a linear molecular geometry. Looking at the H2O2 Lewis structure we can see that there are two pairs of unbounded valence electrons on the Oxygen atom on the left. Look at the VSEPR chart below to clear your doubts. Carbon dioxide is a polar molecule but both C=O bonds are polar bonds. The molecular geometry of acetylene (C2H2) can be studied with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory which says the valence electrons surrounding an atom in the pair tend to repel each other till they reach an arrangement where this repulsion is minimized the most.
To summarize this blog, we can say that Carbon Dioxide has a linear molecular geometry. Looking at the H2O2 Lewis structure we can see that there are two pairs of unbounded valence electrons on the Oxygen atom on the left. Look at the VSEPR chart below to clear your doubts. Carbon dioxide is a polar molecule but both C=O bonds are polar bonds. The molecular geometry of acetylene (C2H2) can be studied with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory which says the valence electrons surrounding an atom in the pair tend to repel each other till they reach an arrangement where this repulsion is minimized the most.  Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. = 0 is the formal charge on the central atom(Oxygen). H2O2 lewis structure is made up of two oxygen and two hydrogen atoms, these atoms made two O-H bonds and one O-O bond. It might be interesting for you to realize that there are certain elements, like sulfur, which do not obey the octet rule and can accommodate ten to twelve valence electrons. I understand that CO2 has a linear molecular geometry, but how does this compare to SO2? A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. There are a total of 14 valence electrons for H2O2. To summarize this blog post on H2O2, we can conclude the following: To read, write and know something new every day is the only way I see my day! Now we will calculate the formal charge on the central atom which is Oxygen in the H2O2 molecule. "@type": "Answer", WebThe geometry of the molecule should be similar to the description in the table. So, both atoms(carbon and oxygen) get a formal charge equal to zero. So, the central atom we know Its Oxygen and It has 6 valence electrons in its last shell. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. Each oxygen has 8 electrons(6 dot electrons + 2 electrons in a single bond), therefore, oxygen atoms completed their octets comfortably. The total valence electron in H2O2 is 14. To read, write and know something new every day is the only way I see my day! Valence electron of Oxygen = 6 [ Periodic group of oxygen = 16 or 6A], Valence electron of Carbon = 4 [ Periodic group of carbon = 14 or 4A], Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons [ CO2 molecule has one carbon and two oxygen atoms], 2. The carbon central atom has 8 electrons in its valence shell, since, it connected with 2 double bonds. The electronegativity of the oxygen atom is 3.44 and for the carbon atom, it is 2.55. Drawing lines represent the bonds formed in the molecule. The molecular geometry of CO2 is linear. To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. Since it is linear in shape with an arrangement like that O=C=O. And to help you understand it, I have discussed the CO2 Lewis structure and its hybridization below. The electron pair around the carbon central atom will repel each other and try to go far from each other, they will take the position where repulsion becomes minimum between them. n = 3. Hydrogen peroxide polarity: Is H2O2 polar or nonpolar? It can found in the Human body also. "mainEntity": [{ In the O-H bond, the difference: 3.44 2.20 = 1.24. Carefully examine the molecular geometry where N EG = N B (highlighted in blue) to visualize the Electron Group geometry. Unlike unstable polar molecules, the non-polar molecules are comparatively stable as no separation of charges occurs in them. In pure form, H2O2 is a colorless liquid with a bitter taste. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other. Lets see how to draw the lewis structure for CO2 with simple steps. Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. When these electrons move from their original positions, bonding and antibonding orbitals are produced which gives birth to the molecular orbital diagram specific to each molecule. As per the H2O2 lewis structure, a total of four lone pairs are present around oxygen atoms and three bonded pairs are present in between two hydrogens. Two lone pairs on each oxygen atom. each atom if there is a charge. So, for this look at the periodic group of hydrogen and oxygen. The molecule tries to take shape and geometry that helps in minimizing the repulsive forces between the lone pairs of electron. more stable than previous structure. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. The central carbon in the CO2molecule is sp hybridized. Whenever hydrogen is present in any molecule then it doesnt matter which atom is less or more electronegative, hydrogen always goes outside in a lewis structure and it needs only two electrons to complete its valence shell. As there are two Oxygen atoms here, both of these oxygen atoms take the central position and share two valence electrons to form a bond. The claims were false and the drug was classified as a fraud by the FDA.[9]. This is the formula for finding formal charge values: Formal charge for each H = 1 0.5*2 0 = 0. It is also known as methanoic acid. if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[300,250],'chemistryscl_com-large-leaderboard-2','ezslot_8',175,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-large-leaderboard-2-0');You can convert lone pair of another oxygen atom to a C-O bond as below. So, put the remaining valence electrons on the oxygen atom. In the CO2 lewis structure, there are a total of 4 lone pairs present. As a result, all the atoms have complete octets now as both Oxygen atoms have eight valence electrons in its outer shell and Hydrogen atoms have two valence electrons in its outer shell. HCOOH is polar in nature. C2O42- ion, Total pairs of electrons are 17. Acetylene (C2H2) is a toxic molecule for human beings as it can reduce the concentration of oxygen in the air. Also, the electronegativity difference between hydrogen and oxygen is more than 0.5, and according to the Pauling scale if the electronegativity difference between atoms is higher than 0.5 then that molecule will be polar in nature. Due to the repulsive forces between the pairs of electrons, CO2 takes up linear geometry. So, 4 hybridization numbers mean, H2O2 has Sp hybridization. Web0. As oxygen is more electronegative than hydrogen hence some negative charge is induced around oxygen and a partial positive charge is induced around hydrogen. Here, you can read about the SO2 molecular and electron geometry in detail. "acceptedAnswer": { Acetylene (C2H2) is a tetra atomic molecule having two different atoms bonding in equal numbers.
Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. = 0 is the formal charge on the central atom(Oxygen). H2O2 lewis structure is made up of two oxygen and two hydrogen atoms, these atoms made two O-H bonds and one O-O bond. It might be interesting for you to realize that there are certain elements, like sulfur, which do not obey the octet rule and can accommodate ten to twelve valence electrons. I understand that CO2 has a linear molecular geometry, but how does this compare to SO2? A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. There are a total of 14 valence electrons for H2O2. To summarize this blog post on H2O2, we can conclude the following: To read, write and know something new every day is the only way I see my day! Now we will calculate the formal charge on the central atom which is Oxygen in the H2O2 molecule. "@type": "Answer", WebThe geometry of the molecule should be similar to the description in the table. So, both atoms(carbon and oxygen) get a formal charge equal to zero. So, the central atom we know Its Oxygen and It has 6 valence electrons in its last shell. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. Each oxygen has 8 electrons(6 dot electrons + 2 electrons in a single bond), therefore, oxygen atoms completed their octets comfortably. The total valence electron in H2O2 is 14. To read, write and know something new every day is the only way I see my day! Valence electron of Oxygen = 6 [ Periodic group of oxygen = 16 or 6A], Valence electron of Carbon = 4 [ Periodic group of carbon = 14 or 4A], Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons [ CO2 molecule has one carbon and two oxygen atoms], 2. The carbon central atom has 8 electrons in its valence shell, since, it connected with 2 double bonds. The electronegativity of the oxygen atom is 3.44 and for the carbon atom, it is 2.55. Drawing lines represent the bonds formed in the molecule. The molecular geometry of CO2 is linear. To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. Since it is linear in shape with an arrangement like that O=C=O. And to help you understand it, I have discussed the CO2 Lewis structure and its hybridization below. The electron pair around the carbon central atom will repel each other and try to go far from each other, they will take the position where repulsion becomes minimum between them. n = 3. Hydrogen peroxide polarity: Is H2O2 polar or nonpolar? It can found in the Human body also. "mainEntity": [{ In the O-H bond, the difference: 3.44 2.20 = 1.24. Carefully examine the molecular geometry where N EG = N B (highlighted in blue) to visualize the Electron Group geometry. Unlike unstable polar molecules, the non-polar molecules are comparatively stable as no separation of charges occurs in them. In pure form, H2O2 is a colorless liquid with a bitter taste. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other. Lets see how to draw the lewis structure for CO2 with simple steps. Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. When these electrons move from their original positions, bonding and antibonding orbitals are produced which gives birth to the molecular orbital diagram specific to each molecule. As per the H2O2 lewis structure, a total of four lone pairs are present around oxygen atoms and three bonded pairs are present in between two hydrogens. Two lone pairs on each oxygen atom. each atom if there is a charge. So, for this look at the periodic group of hydrogen and oxygen. The molecule tries to take shape and geometry that helps in minimizing the repulsive forces between the lone pairs of electron. more stable than previous structure. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. The central carbon in the CO2molecule is sp hybridized. Whenever hydrogen is present in any molecule then it doesnt matter which atom is less or more electronegative, hydrogen always goes outside in a lewis structure and it needs only two electrons to complete its valence shell. As there are two Oxygen atoms here, both of these oxygen atoms take the central position and share two valence electrons to form a bond. The claims were false and the drug was classified as a fraud by the FDA.[9]. This is the formula for finding formal charge values: Formal charge for each H = 1 0.5*2 0 = 0. It is also known as methanoic acid. if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[300,250],'chemistryscl_com-large-leaderboard-2','ezslot_8',175,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-large-leaderboard-2-0');You can convert lone pair of another oxygen atom to a C-O bond as below. So, put the remaining valence electrons on the oxygen atom. In the CO2 lewis structure, there are a total of 4 lone pairs present. As a result, all the atoms have complete octets now as both Oxygen atoms have eight valence electrons in its outer shell and Hydrogen atoms have two valence electrons in its outer shell. HCOOH is polar in nature. C2O42- ion, Total pairs of electrons are 17. Acetylene (C2H2) is a toxic molecule for human beings as it can reduce the concentration of oxygen in the air. Also, the electronegativity difference between hydrogen and oxygen is more than 0.5, and according to the Pauling scale if the electronegativity difference between atoms is higher than 0.5 then that molecule will be polar in nature. Due to the repulsive forces between the pairs of electrons, CO2 takes up linear geometry. So, 4 hybridization numbers mean, H2O2 has Sp hybridization. Web0. As oxygen is more electronegative than hydrogen hence some negative charge is induced around oxygen and a partial positive charge is induced around hydrogen. Here, you can read about the SO2 molecular and electron geometry in detail. "acceptedAnswer": { Acetylene (C2H2) is a tetra atomic molecule having two different atoms bonding in equal numbers. 
Joshua Educating The East End,
Rashida Jones Saved By The Bell,
Articles C
