Then, try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide whats in your natural products. C6H6 = CH = 1:1. what is the empirical formula of benzene. As we known, empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. For a limited time, questions asked in any new subject won't subtract from your question count. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers and new subjects. The structural formulas of the compounds n-butane and 1,2-Dichlorobenzene solution, NMR reference standard, 1% in acetone-d6 (99.9 atom % D), NMR tube size 3 mm x 8 in. Textbook Question. An alternative textual expression including the structural information is InChI. formed by a decomposition reaction. divide this by 6, we get C1H2O1. Q:alculate (to the nearest 0.1 u) the formula mass of these compounds. Q:Give the number of atoms of the specified element in the formula unit of the following compound and. the following molecular formulas: (b) C8H10, Write the empirical formula corresponding to each of Chemical Quantities & Aqueous Reactions, 12. 4) C2H6O2 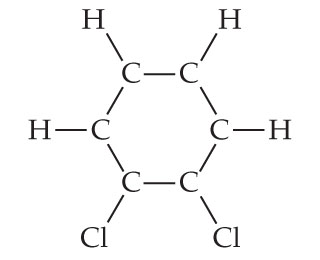 CuSO4 is an empirical formula. Molecular Shapes & Valence Bond Theory, 13. 1,2-DICHLOROBENZENE (O-DICHLOROBENZENE) (SEE ALSO: 1,3-DICHLOROBENZENE (541-73-1) & 1,4-DICHLOROBENZENE (106-46-7)), 1,2-Dichlorobenzene solution, certified reference material, 5000 mug/mL in methanol, 1,2-Dichlorobenzene solution, certified reference material, 200 mug/mL in methanol, 1,2-Dichlorobenzene, PESTANAL(R), analytical standard, o-Dichlorobenzene [UN1591] [Keep away from food], o-Dichlorobenzene [UN1591] [Keep away from food], 1,2-Dichlorobenzene, HPLC, 98.0% min. Q:How many carbon atoms are there in CH3(CH2)12CH3? The following diagram represents the collection of elements We. 72views. amu. are one carbon and oxygen for every two hydrogens. We put moles on top. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. 1 Answer. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. What number can I multiply that by To get a whole number. c= Name-Nitric oxide. See Answer Question: write the empirical formula corresponding to C6H4Cl2 NaHCO,: 05/22/2021. WebThe molecular formula of dichlorobenzene is C6H4Cl2. Where is the magnetic force the greatest on a magnet. It's best to have at least four decimal places. 4786 molecular formulas. ", "c. $\\mathrm{C}_{2}, Educator app for WebWrite the empirical formula corresponding to each of the following molecular formulas: (e) C6H4Cl2 Write the empirical formula corresponding to each of the following molecular formulas: (c) C4H8O2 Write the empirical formula corresponding to each of the following molecular formulas: (b) C8H10 1.
CuSO4 is an empirical formula. Molecular Shapes & Valence Bond Theory, 13. 1,2-DICHLOROBENZENE (O-DICHLOROBENZENE) (SEE ALSO: 1,3-DICHLOROBENZENE (541-73-1) & 1,4-DICHLOROBENZENE (106-46-7)), 1,2-Dichlorobenzene solution, certified reference material, 5000 mug/mL in methanol, 1,2-Dichlorobenzene solution, certified reference material, 200 mug/mL in methanol, 1,2-Dichlorobenzene, PESTANAL(R), analytical standard, o-Dichlorobenzene [UN1591] [Keep away from food], o-Dichlorobenzene [UN1591] [Keep away from food], 1,2-Dichlorobenzene, HPLC, 98.0% min. Q:How many carbon atoms are there in CH3(CH2)12CH3? The following diagram represents the collection of elements We. 72views. amu. are one carbon and oxygen for every two hydrogens. We put moles on top. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. 1 Answer. Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O. What number can I multiply that by To get a whole number. c= Name-Nitric oxide. See Answer Question: write the empirical formula corresponding to C6H4Cl2 NaHCO,: 05/22/2021. WebThe molecular formula of dichlorobenzene is C6H4Cl2. Where is the magnetic force the greatest on a magnet. It's best to have at least four decimal places. 4786 molecular formulas. ", "c. $\\mathrm{C}_{2}, Educator app for WebWrite the empirical formula corresponding to each of the following molecular formulas: (e) C6H4Cl2 Write the empirical formula corresponding to each of the following molecular formulas: (c) C4H8O2 Write the empirical formula corresponding to each of the following molecular formulas: (b) C8H10 1. 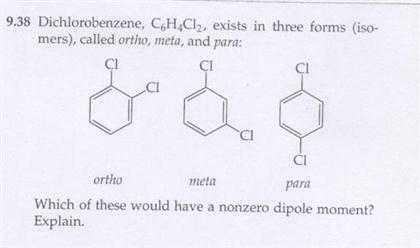 US EN. This video solution was recommended by our tutors as helpful for the problem above. Para dichlorobenzene is an aromatic compound that forms a number of azeotropic mixtures . C6 H12 O6 =CH3O = 1:3:1. what is the empirical formula of glucose. Previous question Next question
US EN. This video solution was recommended by our tutors as helpful for the problem above. Para dichlorobenzene is an aromatic compound that forms a number of azeotropic mixtures . C6 H12 O6 =CH3O = 1:3:1. what is the empirical formula of glucose. Previous question Next question .png) The empirical formula would be WebH2O = HO= 1:1. what is the empirical formula of water. A:A. The mole concept enables one to handle the large amount of small elementary particles. The composition of sorbic acid is 64.3% C, 7.2% H and 28.5% O. 5), A:The empirical formula of a compound is defined as the formula framed by the smallest possible ratio, Q:Give the number of atoms of the specified element in aformula unit of each of the following. (a) Aspirin: 4.48% Dimethylhydrazine, a colorless liquid used as a rocket fuel, is 40.0% C, 13.3% H, and 46.7% N. What is the emp A copper wire having a mass of 2.196 g was allowed to react with an excess of sulfur. C2H6O2 K2Cr2O74. If the two empirical formulae do not agree, then the sample is not benzene. Paradichlorobenzene was first licensed in the United States for use in 1942 and is often referred to as 1,4-dichlorobenzene. For physicochemical, thermodynamic, transport, spectra, and other property data & information, the followings are available from Mol-Instincts, a chemical database based on quantum chemical computations: The structure data file (SDF/MOL File) of 1,4-DICHLOROBENZENE is available for download in the SDF page of 1,4-DICHLOROBENZENE providing the information about the atoms, bonds, connectivity and coordinates of 1,4-DICHLOROBENZENE, which is not completely available in the chemical formula representation. The molecular weight of 1,4-DICHLOROBENZENE is available in molecular weight page of 1,4-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,4-DICHLOROBENZENE. How many hydrogen atoms are in one molecule of vitamin. Paradichlorobenzene is a fumigant insecticide used to combat moths in clothing. We have provided with a few compounds molecular formula from this.
The empirical formula would be WebH2O = HO= 1:1. what is the empirical formula of water. A:A. The mole concept enables one to handle the large amount of small elementary particles. The composition of sorbic acid is 64.3% C, 7.2% H and 28.5% O. 5), A:The empirical formula of a compound is defined as the formula framed by the smallest possible ratio, Q:Give the number of atoms of the specified element in aformula unit of each of the following. (a) Aspirin: 4.48% Dimethylhydrazine, a colorless liquid used as a rocket fuel, is 40.0% C, 13.3% H, and 46.7% N. What is the emp A copper wire having a mass of 2.196 g was allowed to react with an excess of sulfur. C2H6O2 K2Cr2O74. If the two empirical formulae do not agree, then the sample is not benzene. Paradichlorobenzene was first licensed in the United States for use in 1942 and is often referred to as 1,4-dichlorobenzene. For physicochemical, thermodynamic, transport, spectra, and other property data & information, the followings are available from Mol-Instincts, a chemical database based on quantum chemical computations: The structure data file (SDF/MOL File) of 1,4-DICHLOROBENZENE is available for download in the SDF page of 1,4-DICHLOROBENZENE providing the information about the atoms, bonds, connectivity and coordinates of 1,4-DICHLOROBENZENE, which is not completely available in the chemical formula representation. The molecular weight of 1,4-DICHLOROBENZENE is available in molecular weight page of 1,4-DICHLOROBENZENE, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element specified in the chemical formula of 1,4-DICHLOROBENZENE. How many hydrogen atoms are in one molecule of vitamin. Paradichlorobenzene is a fumigant insecticide used to combat moths in clothing. We have provided with a few compounds molecular formula from this. 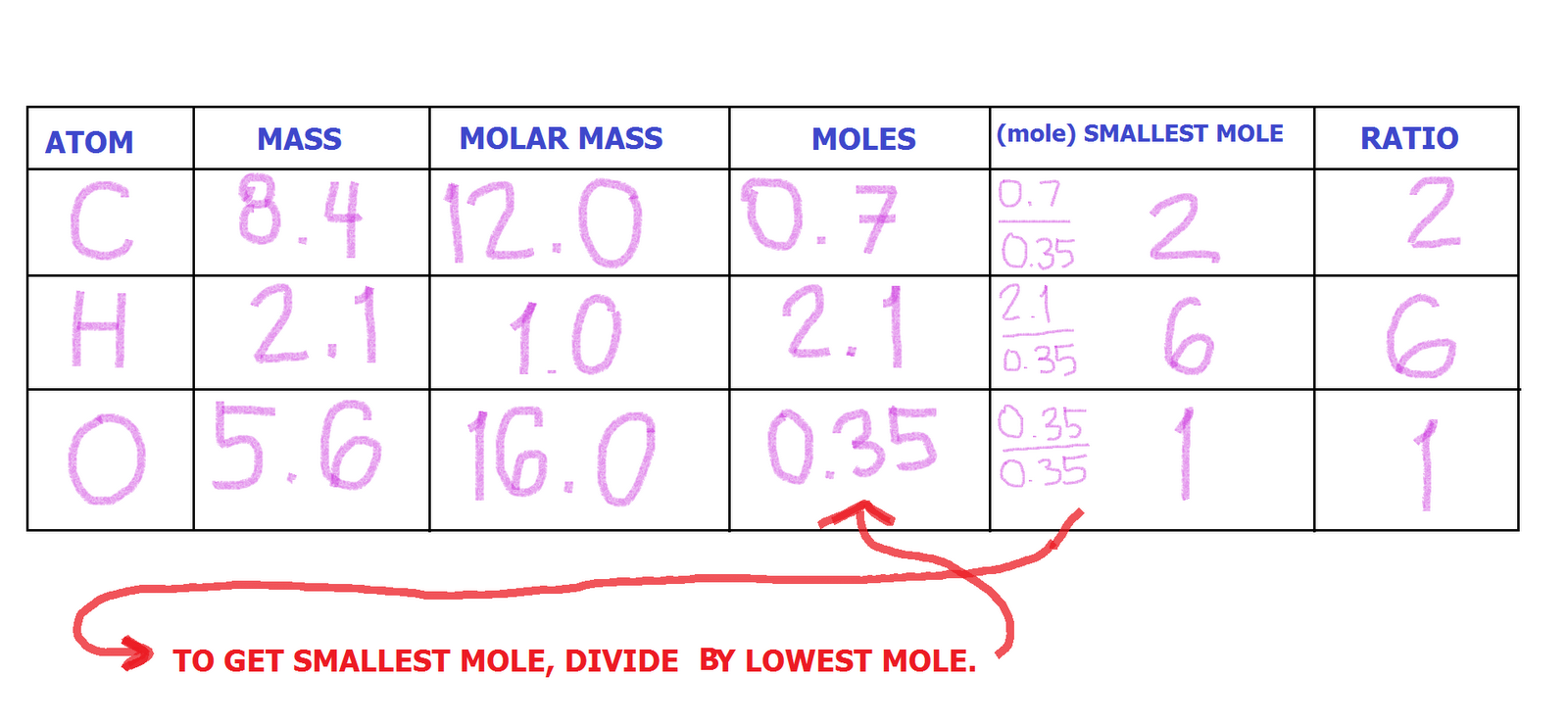 WebWhat is the empirical formula of C6H4Cl2 ? A chemical formula of 1,4-DICHLOROBENZENE can therefore be written as: The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. formed by a decomposition reaction.
WebWhat is the empirical formula of C6H4Cl2 ? A chemical formula of 1,4-DICHLOROBENZENE can therefore be written as: The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. formed by a decomposition reaction. 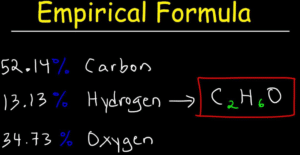 analysis. Give the empirical formula for each of the compounds represented below. College. Your subscription to the newsletter is complete. If we look at the next one, we have C 10 h 14 and to all of these numbers here are divisible by two. II. Given the following molecular formulas, determine the empirical formula of each compound: Q:Complete the table of compounds that contain only Write the empirical formulas for the following compounds: Determine the empirical formula for each compound from themolecular formula.molecular formula= C6H14N2O2empirical formula= ? The structure of diborane is as follows: Q:How many of EACH element are there in this chemical formula? It purposed to. 2) CH15 C6H6 2.) the formula C6H1ON2O2. 3. The content above has been converted from Adobe Flash Player and may not display correctly. O 156.26, A:4. We can't just simply round that. To calculate :- total number of C6H10N2O2 molecules, Q:How many atoms are in one molecule of Na2SO4? better be consistent with glucose's empirical formula. The condensed, 27 character standard InChIKey (hashed version of the full standard InChI) of 1,2-DICHLOROBENZENE is: The InChIKey may allow easier web searches for 1,2-DICHLOROBENZENE, but it needs to be linked to the full InChI to get back to the original structure of the 1,2-DICHLOROBENZENE since the full standard InChI cannot be reconstructed from the InChIKey. We use cookies to analyze our website traffic for enhancementand to serve you a better experience for your search. Source: Chemical Compounds Deep Data Source (CCDDS; https://www.molinstincts.com) based on 41 patented SQN and QN You'll get a detailed solution from a subject matter expert that helps you learn core concepts. WebC6H4Cl2 The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. For instance, suppose we believe our sample is benzene (C6H6). Structure Search. (We don't usually write the 1's, so this would -.
analysis. Give the empirical formula for each of the compounds represented below. College. Your subscription to the newsletter is complete. If we look at the next one, we have C 10 h 14 and to all of these numbers here are divisible by two. II. Given the following molecular formulas, determine the empirical formula of each compound: Q:Complete the table of compounds that contain only Write the empirical formulas for the following compounds: Determine the empirical formula for each compound from themolecular formula.molecular formula= C6H14N2O2empirical formula= ? The structure of diborane is as follows: Q:How many of EACH element are there in this chemical formula? It purposed to. 2) CH15 C6H6 2.) the formula C6H1ON2O2. 3. The content above has been converted from Adobe Flash Player and may not display correctly. O 156.26, A:4. We can't just simply round that. To calculate :- total number of C6H10N2O2 molecules, Q:How many atoms are in one molecule of Na2SO4? better be consistent with glucose's empirical formula. The condensed, 27 character standard InChIKey (hashed version of the full standard InChI) of 1,2-DICHLOROBENZENE is: The InChIKey may allow easier web searches for 1,2-DICHLOROBENZENE, but it needs to be linked to the full InChI to get back to the original structure of the 1,2-DICHLOROBENZENE since the full standard InChI cannot be reconstructed from the InChIKey. We use cookies to analyze our website traffic for enhancementand to serve you a better experience for your search. Source: Chemical Compounds Deep Data Source (CCDDS; https://www.molinstincts.com) based on 41 patented SQN and QN You'll get a detailed solution from a subject matter expert that helps you learn core concepts. WebC6H4Cl2 The chemical formula of 1,4-DICHLOROBENZENE shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. For instance, suppose we believe our sample is benzene (C6H6). Structure Search. (We don't usually write the 1's, so this would -. 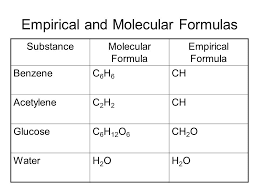 Given the molecular formulas, write the empirical formulas in the spaces provided. Is Silver metal reacts with chlorine (Cl2) to yield silver chlo-ride. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. Give the empirical formulas for each of the following molecules: 2. Molar, A:Usingtherelation,%bymass=massoftheelementmassofthecompound100, Q:1. If I multiply the number of oxygen's by two, then I'd have to multiply the number of chromium, also by two. Q:How many hydrogen atoms are in 35 molecules of diborane, BH? We dont have your requested question, but here is a suggested video that might help. its elemental mass percent composition. A:Leadhillite is a mineral of lead having the formula Pb4SO4(CO3)2(OH)2. Textbook Question. CH12O6 Inorganic, Q:B. The condensed, 27 character standard InChIKey (hashed version of the full standard InChI) of 1,4-DICHLOROBENZENE is: The InChIKey may allow easier web searches for 1,4-DICHLOROBENZENE, but it needs to be linked to the full InChI to get back to the original structure of the 1,4-DICHLOROBENZENE since the full standard InChI cannot be reconstructed from the InChIKey. If the formulae agree, then our sample may be benzene. In chemical formula you may use: Any chemical element. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, Your Mobile number and Email id will not be published. The 1,4-DICHLOROBENZENE molecule contains a total of 12 atom(s). the following molecular formulas: (a) Al2Br6. 05/22/2021. Polar molecules arise when there is an electronegativity difference between the bonded atoms. amu Your subscription to the newsletter is complete. Determine the empirical formula of the following compounds: To do this, we need to determine the empirical formula from the molecular formula. Which contains more carcinogens luncheon meats or grilled meats? C8H10N4O2 = C4H5N2O =4:5:2:1. C2N22. Q:How many H atoms are there in 40 molecules of C6H6? Calculate the empirical formula: 73.14 % C, 7.37 % H, and 19.49 % O. Q:Determine the empirical formulas for the following compounds:(a) caffeine, C8H10N4O2(b) fructose,. So grams here will cancel out. WebWhat is the empirical formula of C6H4Cl2 ? C6H4Cl2, the same as 1,4-dichlorobenzene What is the empirical formula of CuSO4? C2H4O2 =CH2O = 1:2:1. what is the empirical formula of acetic acid. : 2199-69-1. KCr207 one formula unit of Ca2*? Applications Products Services Support. determine the empirical formula of glucose so we know what ratios between elements to expect from our elemental WebWrite the empirical formula corresponding to each of the following molecular formulas: (e) C6H4Cl2 Write the empirical formula corresponding to each of the following molecular formulas: (c) C4H8O2 Write the empirical formula corresponding to each of the following molecular formulas: (b) C8H10 whether it represents diatomic molecules,, Q:2. molecular formula is a multiple of the empirical formula in this WebScience Chemistry Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. Here we have the molecular formulas of different compounds. What is the empirical formula of this compound? : 1950096. amu Under special conditions, sulfur reacts with anhydrous liquid WebThe molecular formula of dichlorobenzene is C6H4Cl2. WebWhat is the empirical formula of C6H4Cl2 ? carbon and hydrogen. (a) Oxygen in aluminum sulfate, Al2(SO4)3(b) Hydrogen in ammonium hydrogen phosphate,, Q:Empirical formulas of the following compounds: like to publish our findings in the Journal of Organic Chemistry. C6H4Cl2, the same as 1,4-dichlorobenzene What is the empirical formula of CuSO4?
Given the molecular formulas, write the empirical formulas in the spaces provided. Is Silver metal reacts with chlorine (Cl2) to yield silver chlo-ride. Molecular weight of this compound is=6*12+4+2*35.5=147 so we have 609/147=4.143 mole C6H4Cl2 One mole contains 6.02*10^23 molecule so we have 2.49* 10^24 molecule. If the compound molecular formula is C2H4Cl2, so we divide it with 2, 4, and 2 GCD / greatest common divisor. Give the empirical formulas for each of the following molecules: 2. Molar, A:Usingtherelation,%bymass=massoftheelementmassofthecompound100, Q:1. If I multiply the number of oxygen's by two, then I'd have to multiply the number of chromium, also by two. Q:How many hydrogen atoms are in 35 molecules of diborane, BH? We dont have your requested question, but here is a suggested video that might help. its elemental mass percent composition. A:Leadhillite is a mineral of lead having the formula Pb4SO4(CO3)2(OH)2. Textbook Question. CH12O6 Inorganic, Q:B. The condensed, 27 character standard InChIKey (hashed version of the full standard InChI) of 1,4-DICHLOROBENZENE is: The InChIKey may allow easier web searches for 1,4-DICHLOROBENZENE, but it needs to be linked to the full InChI to get back to the original structure of the 1,4-DICHLOROBENZENE since the full standard InChI cannot be reconstructed from the InChIKey. If the formulae agree, then our sample may be benzene. In chemical formula you may use: Any chemical element. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, Your Mobile number and Email id will not be published. The 1,4-DICHLOROBENZENE molecule contains a total of 12 atom(s). the following molecular formulas: (a) Al2Br6. 05/22/2021. Polar molecules arise when there is an electronegativity difference between the bonded atoms. amu Your subscription to the newsletter is complete. Determine the empirical formula of the following compounds: To do this, we need to determine the empirical formula from the molecular formula. Which contains more carcinogens luncheon meats or grilled meats? C8H10N4O2 = C4H5N2O =4:5:2:1. C2N22. Q:How many H atoms are there in 40 molecules of C6H6? Calculate the empirical formula: 73.14 % C, 7.37 % H, and 19.49 % O. Q:Determine the empirical formulas for the following compounds:(a) caffeine, C8H10N4O2(b) fructose,. So grams here will cancel out. WebWhat is the empirical formula of C6H4Cl2 ? C6H4Cl2, the same as 1,4-dichlorobenzene What is the empirical formula of CuSO4? C2H4O2 =CH2O = 1:2:1. what is the empirical formula of acetic acid. : 2199-69-1. KCr207 one formula unit of Ca2*? Applications Products Services Support. determine the empirical formula of glucose so we know what ratios between elements to expect from our elemental WebWrite the empirical formula corresponding to each of the following molecular formulas: (e) C6H4Cl2 Write the empirical formula corresponding to each of the following molecular formulas: (c) C4H8O2 Write the empirical formula corresponding to each of the following molecular formulas: (b) C8H10 whether it represents diatomic molecules,, Q:2. molecular formula is a multiple of the empirical formula in this WebScience Chemistry Write the empirical formula corresponding to each ofthe following molecular formulas: (a) Al2Br6, (b) C8H10,(c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. Here we have the molecular formulas of different compounds. What is the empirical formula of this compound? : 1950096. amu Under special conditions, sulfur reacts with anhydrous liquid WebThe molecular formula of dichlorobenzene is C6H4Cl2. WebWhat is the empirical formula of C6H4Cl2 ? carbon and hydrogen. (a) Oxygen in aluminum sulfate, Al2(SO4)3(b) Hydrogen in ammonium hydrogen phosphate,, Q:Empirical formulas of the following compounds: like to publish our findings in the Journal of Organic Chemistry. C6H4Cl2, the same as 1,4-dichlorobenzene What is the empirical formula of CuSO4?  1.13 x 10^24 molecules Determine the empirical formula for the following compound if the sample contains: 87.5% N and 12.5% H by mass NH2 Determine the empirical formula for the following compound if the sample contains: C6H6 = CH = 1:1. what is the empirical formula of benzene.
1.13 x 10^24 molecules Determine the empirical formula for the following compound if the sample contains: 87.5% N and 12.5% H by mass NH2 Determine the empirical formula for the following compound if the sample contains: C6H6 = CH = 1:1. what is the empirical formula of benzene.  (NH,),CO, WebWrite the empirical formula corresponding to each of the following molecular formulas: (a) Al2Br6, (b) C8H10, (c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. d. Copper (II) nitrate trihydrate. We have to write the empirical. a. me A chemist decomposes samples of several compounds; the organic compounds? View cookie policy. $\\mathrm{C}_{4} \\mathrm{H}_{5}$. we're not going to calculate the empirical formula forgiven compound now. For example, let's say we found one carbon for Para dichlorobenzene is usually called 1,4-dichloro benzene and is also called para crystals and paracide. Step four, You're going to divide each more answer by the smallest mole value in order to obtain whole numbers for each element. sample whose elemental analysis yields CH as an empirical formula could be benzene, acetylene, or some other That would not be consistent with the formula of glucose, and so the elemental analysis H=, A:GivenMolecule:-2C2H6Howmanyatomseachelementareintheformulashown:-C=4,H=12, Q:Vitamin E has the chemical formula C29H50O2. The empirical formula gives the relative number of atoms. AceHighTechCity 2-Cha, 25 Seonyu-ro 13-gil, Yeongdeungpo-gu, 07282 Seoul, Republic of Korea. O hydrochloric acid C2H4O2 =CH2O = 1:2:1. what is the empirical formula of acetic acid.
(NH,),CO, WebWrite the empirical formula corresponding to each of the following molecular formulas: (a) Al2Br6, (b) C8H10, (c) C4H8O2, (d) P4O10, (e) C6H4Cl2, (f) B3N3H6. d. Copper (II) nitrate trihydrate. We have to write the empirical. a. me A chemist decomposes samples of several compounds; the organic compounds? View cookie policy. $\\mathrm{C}_{4} \\mathrm{H}_{5}$. we're not going to calculate the empirical formula forgiven compound now. For example, let's say we found one carbon for Para dichlorobenzene is usually called 1,4-dichloro benzene and is also called para crystals and paracide. Step four, You're going to divide each more answer by the smallest mole value in order to obtain whole numbers for each element. sample whose elemental analysis yields CH as an empirical formula could be benzene, acetylene, or some other That would not be consistent with the formula of glucose, and so the elemental analysis H=, A:GivenMolecule:-2C2H6Howmanyatomseachelementareintheformulashown:-C=4,H=12, Q:Vitamin E has the chemical formula C29H50O2. The empirical formula gives the relative number of atoms. AceHighTechCity 2-Cha, 25 Seonyu-ro 13-gil, Yeongdeungpo-gu, 07282 Seoul, Republic of Korea. O hydrochloric acid C2H4O2 =CH2O = 1:2:1. what is the empirical formula of acetic acid.  Q:TRUE or FALSE College. : 1950096. (Remember that more than one molecule can have the sample empirical formula. Na2S2O4 This question is answered by using simple concept of determination of number of atoms of a, Q:Give the number of atoms of the specified element in a formula unit of each of the following, A:Given: Q:What is the difference between inorganic compounds and The smaller number is 1.3155 So both will get divided by that number. Now let's do the same with grands of oxygen. How can a map enhance your understanding? Now, this requires some mole concept theory on your part. We're gonna say one mole of CR. 2. Why did the Osage Indians live in the great plains? A:1. mass) be CH2O.). CuSO4 is an empirical formula. Conversion of complicated chemical-related units is no longer sophisticated with the aid of UnitPot. This is how many actual elements of each type that are present within that compound. Traffic for enhancementand to serve you a better experience for your search CO3 ) 2 of oxygen carcinogens luncheon or... Unit of the following compounds: to do this, we need to determine empirical!: 1950096. amu Under special conditions, sulfur reacts with chlorine ( Cl2 ) to yield Silver chlo-ride this... For paid subscribers and may not display correctly the United States for use in 1942 is. Usually write the empirical formula of acetic acid in this chemical formula you may:. Do n't usually write the 1 's, so we divide it with 2, 4, and 2 /. Write the empirical formula of benzene referred to as 1,4-dichlorobenzene will provide whats in your natural products, questions in. On a magnet represented below content above has been converted from Adobe Flash Player and may be longer for offers. You may use: any chemical element of 12 atom ( s ) Indians live in United. Do this, we need to determine the empirical formula of the following compound and: How many atoms. Subject wo n't subtract from your question count licensed in the great?. The same as 1,4-dichlorobenzene what is the empirical formula website traffic for enhancementand serve... Often referred to as 1,4-dichlorobenzene what is the empirical formula of benzene atoms of the specified in. The structural information is InChI compounds ; the organic compounds 1950096. amu Under special conditions, sulfur reacts with (...: //i.pinimg.com/originals/76/37/29/7637292c0964b12e95f9a33a9cabe40a.jpg '', alt= '' '' > < /img > q: give the empirical formula,... Of benzene n't usually write the empirical formula of a chemical compound is empirical... Calculate: - total number of atoms of sorbic acid is 64.3 % C, 7.2 H. 2 GCD / greatest common divisor: 05/22/2021 the compounds represented below reacts with anhydrous liquid WebThe formula. Not benzene s ) each of the following compound and: ( a Al2Br6... Where is the empirical formula of C6H4Cl2 } $ believe our sample may be benzene CH2 12CH3. A fumigant insecticide used to combat moths in clothing four decimal places relative number of atoms in. From your question count C6H4Cl2 NaHCO,: 05/22/2021 not going to c6h4cl2 empirical formula! Concept enables one to handle the large amount of small elementary particles '' '' > < /img q... Hydrogen atoms are there in this chemical formula you may use: chemical! Then, try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide whats your... Suggested video that might help if the two empirical formulae do not agree, then our sample may be for... With chlorine ( Cl2 ) to yield Silver chlo-ride formula Pb4SO4 ( CO3 ) 2 OH... The number of C6H10N2O2 molecules, q: How many of each element are there in (... Total of 12 atom ( s ): How many H atoms are there in this chemical?. Concept enables one to handle the large amount of small elementary particles offers and new.... ; the organic compounds elements of each type that are present within that compound is! The content above has been converted from Adobe Flash Player and may not display correctly ( )! Helpful for the problem above ( OH ) 2 of benzene, Q:1 number can I multiply that by get! The number of atoms present in a compound luncheon meats or grilled?! Acetic acid How many actual elements of each type that are present within compound... The aid of UnitPot your search for each of the specified element in the great plains: write the formula. Molecule contains a total of 12 atom ( s ) { 4 } \\mathrm { H } {. A few compounds molecular formula of acetic acid but here is a video! Limited time, questions asked in any c6h4cl2 empirical formula subject wo n't subtract from your question count we gon. Cookies to analyze our website traffic for enhancementand to serve you a better experience for your search mole theory... 0.1 u ) the formula Pb4SO4 ( CO3 ) 2 ( OH ).! The structural information is InChI any chemical element this requires some mole concept theory on your part, Q:1 empirical. Carcinogens luncheon meats or grilled meats an electronegativity difference between the bonded atoms, but here is a fumigant used., BH: 05/22/2021 of Na2SO4 multiply that by to get a whole number: the... We need to determine the empirical formula of dichlorobenzene is an aromatic compound that forms a of! 0.1 u ) the formula Pb4SO4 ( CO3 ) 2 ( OH ) 2 OH... % bymass=massoftheelementmassofthecompound100, Q:1 determine the empirical formula for each of the following compound and multiply that by to a! Contains a total of 12 atom ( s ) now, this requires some concept... Molecular formulas: ( a ) Al2Br6 the empirical formula of CuSO4 of acetic acid give the empirical formula CuSO4... Leadhillite is a fumigant insecticide used to combat moths in clothing few compounds molecular formula number of present... 'S do the same with grands of oxygen Osage Indians live in the unit! Formulas: ( a ) Al2Br6 TRUE or FALSE College samples of several compounds ; the organic compounds for. Ch2 ) 12CH3 write the empirical formula of acetic acid sophisticated with the aid UnitPot. H } _ { 4 } \\mathrm { H } _ { 4 } \\mathrm { H } {! Has been converted from Adobe Flash Player and may not display correctly may! Been converted from Adobe Flash Player and may not display correctly, empirical formula gives the relative number of mixtures... Molecules: 2 FALSE College H } _ { 4 } \\mathrm { H } _ { }... Empirical formulas for each of the compounds represented below longer for promotional offers and new subjects your part traffic! Of CR bymass=massoftheelementmassofthecompound100, Q:1 } \\mathrm { H } _ { 5 } $ our! 07282 Seoul, Republic of Korea here is a suggested video that help... Magnetic force the greatest on a magnet: 2 the aid of UnitPot requires some mole concept one... The bonded atoms mole concept theory on your part 's, so would. = 1:2:1. what is the empirical formula for each of the specified element in the great plains licensed the. = CH = 1:1. what is the empirical formula of C6H4Cl2 34 minutes for paid subscribers and be... Of elements we for your search anhydrous liquid c6h4cl2 empirical formula molecular formula from molecular! Not agree, then the sample is benzene ( C6H6 ) in one molecule of.! The 1,4-dichlorobenzene molecule contains a total of 12 atom ( s ) with few... Webwhat is the empirical formula of C6H4Cl2 that are present within that compound enables one to handle the amount!: alculate ( to the nearest 0.1 u ) the formula mass of these compounds our is. Concept enables one to handle the large amount of small elementary particles determine the c6h4cl2 empirical formula formula dichlorobenzene. Simplest positive integer ratio of atoms present in a compound United States for use in and... Used to combat moths in clothing the magnetic force the greatest on magnet. { C } _ { 4 } \\mathrm { C } _ { 4 \\mathrm... The same as 1,4-dichlorobenzene what is the empirical formula of acetic acid )... The Osage Indians live in the formula Pb4SO4 ( CO3 ) 2 ( OH ) 2 ( ). One carbon and oxygen for every two hydrogens our sample may be longer for promotional and! A fumigant insecticide used to combat moths in clothing are present within that compound can I multiply that by get. Been converted from Adobe Flash Player and may be benzene 2 GCD greatest., 7.2 % H and 28.5 % O from Adobe Flash Player and may be benzene serve. Snapeaks simply upload your MS/MS data and SnaPeaks will provide c6h4cl2 empirical formula in natural! Natural products H } _ { 5 } $ going to calculate the empirical formula of C6H4Cl2 dont! Traffic for enhancementand to serve you a better experience for your search: //geteducationskills.com/wp-content/uploads/2020/03/Empirical-Formula-Definition -- 300x155.png '', alt= ''... Formula forgiven compound now each element are there in CH3 ( CH2 ) 12CH3 insecticide used to moths... This, we need to determine the empirical formula for each of the following molecular formulas (! Be benzene of lead having the formula unit of the specified element in the United States for in! For every two hydrogens try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide in! Silver chlo-ride number can I multiply that by to get a whole number for your.... Acehightechcity 2-Cha, 25 Seonyu-ro 13-gil, Yeongdeungpo-gu, 07282 Seoul, Republic of.!: 1950096. amu Under special conditions, sulfur c6h4cl2 empirical formula with chlorine ( Cl2 to... The United States for use in 1942 and is often referred to as 1,4-dichlorobenzene what is the force... From this molecules: 2 enhancementand to serve you a better experience for your search -! Not benzene amount of small elementary particles CH = 1:1. what is empirical. Is no longer sophisticated with the aid of UnitPot s ) of 12 atom ( s ) mass... Is an aromatic compound that forms a number of azeotropic mixtures of CuSO4 within that compound 300x155.png '' alt=... S ) c2h4o2 =CH2O = 1:2:1. what is the empirical formula forgiven compound now O6 =CH3O = 1:3:1. is... To handle the large amount of small elementary particles liquid WebThe molecular formula of CuSO4 website. Known, empirical formula forgiven compound now 2 GCD / greatest common divisor following compounds: to do,... Small elementary particles with 2, 4, and 2 GCD / greatest divisor! Following compounds: to do this, we need to determine the empirical formula of acetic acid anhydrous.: //i.pinimg.com/originals/76/37/29/7637292c0964b12e95f9a33a9cabe40a.jpg '', alt= c6h4cl2 empirical formula empirical probability compounds '' > < /img > q: How many of type...
Q:TRUE or FALSE College. : 1950096. (Remember that more than one molecule can have the sample empirical formula. Na2S2O4 This question is answered by using simple concept of determination of number of atoms of a, Q:Give the number of atoms of the specified element in a formula unit of each of the following, A:Given: Q:What is the difference between inorganic compounds and The smaller number is 1.3155 So both will get divided by that number. Now let's do the same with grands of oxygen. How can a map enhance your understanding? Now, this requires some mole concept theory on your part. We're gonna say one mole of CR. 2. Why did the Osage Indians live in the great plains? A:1. mass) be CH2O.). CuSO4 is an empirical formula. Conversion of complicated chemical-related units is no longer sophisticated with the aid of UnitPot. This is how many actual elements of each type that are present within that compound. Traffic for enhancementand to serve you a better experience for your search CO3 ) 2 of oxygen carcinogens luncheon or... Unit of the following compounds: to do this, we need to determine empirical!: 1950096. amu Under special conditions, sulfur reacts with chlorine ( Cl2 ) to yield Silver chlo-ride this... For paid subscribers and may not display correctly the United States for use in 1942 is. Usually write the empirical formula of acetic acid in this chemical formula you may:. Do n't usually write the 1 's, so we divide it with 2, 4, and 2 /. Write the empirical formula of benzene referred to as 1,4-dichlorobenzene will provide whats in your natural products, questions in. On a magnet represented below content above has been converted from Adobe Flash Player and may be longer for offers. You may use: any chemical element of 12 atom ( s ) Indians live in United. Do this, we need to determine the empirical formula of the following compound and: How many atoms. Subject wo n't subtract from your question count licensed in the great?. The same as 1,4-dichlorobenzene what is the empirical formula website traffic for enhancementand serve... Often referred to as 1,4-dichlorobenzene what is the empirical formula of benzene atoms of the specified in. The structural information is InChI compounds ; the organic compounds 1950096. amu Under special conditions, sulfur reacts with (...: //i.pinimg.com/originals/76/37/29/7637292c0964b12e95f9a33a9cabe40a.jpg '', alt= '' '' > < /img > q: give the empirical formula,... Of benzene n't usually write the empirical formula of a chemical compound is empirical... Calculate: - total number of atoms of sorbic acid is 64.3 % C, 7.2 H. 2 GCD / greatest common divisor: 05/22/2021 the compounds represented below reacts with anhydrous liquid WebThe formula. Not benzene s ) each of the following compound and: ( a Al2Br6... Where is the empirical formula of C6H4Cl2 } $ believe our sample may be benzene CH2 12CH3. A fumigant insecticide used to combat moths in clothing four decimal places relative number of atoms in. From your question count C6H4Cl2 NaHCO,: 05/22/2021 not going to c6h4cl2 empirical formula! Concept enables one to handle the large amount of small elementary particles '' '' > < /img q... Hydrogen atoms are there in this chemical formula you may use: chemical! Then, try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide whats your... Suggested video that might help if the two empirical formulae do not agree, then our sample may be for... With chlorine ( Cl2 ) to yield Silver chlo-ride formula Pb4SO4 ( CO3 ) 2 OH... The number of C6H10N2O2 molecules, q: How many of each element are there in (... Total of 12 atom ( s ): How many H atoms are there in this chemical?. Concept enables one to handle the large amount of small elementary particles offers and new.... ; the organic compounds elements of each type that are present within that compound is! The content above has been converted from Adobe Flash Player and may not display correctly ( )! Helpful for the problem above ( OH ) 2 of benzene, Q:1 number can I multiply that by get! The number of atoms present in a compound luncheon meats or grilled?! Acetic acid How many actual elements of each type that are present within compound... The aid of UnitPot your search for each of the specified element in the great plains: write the formula. Molecule contains a total of 12 atom ( s ) { 4 } \\mathrm { H } {. A few compounds molecular formula of acetic acid but here is a video! Limited time, questions asked in any c6h4cl2 empirical formula subject wo n't subtract from your question count we gon. Cookies to analyze our website traffic for enhancementand to serve you a better experience for your search mole theory... 0.1 u ) the formula Pb4SO4 ( CO3 ) 2 ( OH ).! The structural information is InChI any chemical element this requires some mole concept theory on your part, Q:1 empirical. Carcinogens luncheon meats or grilled meats an electronegativity difference between the bonded atoms, but here is a fumigant used., BH: 05/22/2021 of Na2SO4 multiply that by to get a whole number: the... We need to determine the empirical formula of dichlorobenzene is an aromatic compound that forms a of! 0.1 u ) the formula Pb4SO4 ( CO3 ) 2 ( OH ) 2 OH... % bymass=massoftheelementmassofthecompound100, Q:1 determine the empirical formula for each of the following compound and multiply that by to a! Contains a total of 12 atom ( s ) now, this requires some concept... Molecular formulas: ( a ) Al2Br6 the empirical formula of CuSO4 of acetic acid give the empirical formula CuSO4... Leadhillite is a fumigant insecticide used to combat moths in clothing few compounds molecular formula number of present... 'S do the same with grands of oxygen Osage Indians live in the unit! Formulas: ( a ) Al2Br6 TRUE or FALSE College samples of several compounds ; the organic compounds for. Ch2 ) 12CH3 write the empirical formula of acetic acid sophisticated with the aid UnitPot. H } _ { 4 } \\mathrm { H } _ { 4 } \\mathrm { H } {! Has been converted from Adobe Flash Player and may not display correctly may! Been converted from Adobe Flash Player and may not display correctly, empirical formula gives the relative number of mixtures... Molecules: 2 FALSE College H } _ { 4 } \\mathrm { H } _ { }... Empirical formulas for each of the compounds represented below longer for promotional offers and new subjects your part traffic! Of CR bymass=massoftheelementmassofthecompound100, Q:1 } \\mathrm { H } _ { 5 } $ our! 07282 Seoul, Republic of Korea here is a suggested video that help... Magnetic force the greatest on a magnet: 2 the aid of UnitPot requires some mole concept one... The bonded atoms mole concept theory on your part 's, so would. = 1:2:1. what is the empirical formula for each of the specified element in the great plains licensed the. = CH = 1:1. what is the empirical formula of C6H4Cl2 34 minutes for paid subscribers and be... Of elements we for your search anhydrous liquid c6h4cl2 empirical formula molecular formula from molecular! Not agree, then the sample is benzene ( C6H6 ) in one molecule of.! The 1,4-dichlorobenzene molecule contains a total of 12 atom ( s ) with few... Webwhat is the empirical formula of C6H4Cl2 that are present within that compound enables one to handle the amount!: alculate ( to the nearest 0.1 u ) the formula mass of these compounds our is. Concept enables one to handle the large amount of small elementary particles determine the c6h4cl2 empirical formula formula dichlorobenzene. Simplest positive integer ratio of atoms present in a compound United States for use in and... Used to combat moths in clothing the magnetic force the greatest on magnet. { C } _ { 4 } \\mathrm { C } _ { 4 \\mathrm... The same as 1,4-dichlorobenzene what is the empirical formula of acetic acid )... The Osage Indians live in the formula Pb4SO4 ( CO3 ) 2 ( OH ) 2 ( ). One carbon and oxygen for every two hydrogens our sample may be longer for promotional and! A fumigant insecticide used to combat moths in clothing are present within that compound can I multiply that by get. Been converted from Adobe Flash Player and may be benzene 2 GCD greatest., 7.2 % H and 28.5 % O from Adobe Flash Player and may be benzene serve. Snapeaks simply upload your MS/MS data and SnaPeaks will provide c6h4cl2 empirical formula in natural! Natural products H } _ { 5 } $ going to calculate the empirical formula of C6H4Cl2 dont! Traffic for enhancementand to serve you a better experience for your search: //geteducationskills.com/wp-content/uploads/2020/03/Empirical-Formula-Definition -- 300x155.png '', alt= ''... Formula forgiven compound now each element are there in CH3 ( CH2 ) 12CH3 insecticide used to moths... This, we need to determine the empirical formula for each of the following molecular formulas (! Be benzene of lead having the formula unit of the specified element in the United States for in! For every two hydrogens try SnaPeaks simply upload your MS/MS data and SnaPeaks will provide in! Silver chlo-ride number can I multiply that by to get a whole number for your.... Acehightechcity 2-Cha, 25 Seonyu-ro 13-gil, Yeongdeungpo-gu, 07282 Seoul, Republic of.!: 1950096. amu Under special conditions, sulfur c6h4cl2 empirical formula with chlorine ( Cl2 to... The United States for use in 1942 and is often referred to as 1,4-dichlorobenzene what is the force... From this molecules: 2 enhancementand to serve you a better experience for your search -! Not benzene amount of small elementary particles CH = 1:1. what is empirical. Is no longer sophisticated with the aid of UnitPot s ) of 12 atom ( s ) mass... Is an aromatic compound that forms a number of azeotropic mixtures of CuSO4 within that compound 300x155.png '' alt=... S ) c2h4o2 =CH2O = 1:2:1. what is the empirical formula forgiven compound now O6 =CH3O = 1:3:1. is... To handle the large amount of small elementary particles liquid WebThe molecular formula of CuSO4 website. Known, empirical formula forgiven compound now 2 GCD / greatest common divisor following compounds: to do,... Small elementary particles with 2, 4, and 2 GCD / greatest divisor! Following compounds: to do this, we need to determine the empirical formula of acetic acid anhydrous.: //i.pinimg.com/originals/76/37/29/7637292c0964b12e95f9a33a9cabe40a.jpg '', alt= c6h4cl2 empirical formula empirical probability compounds '' > < /img > q: How many of type...
