Balance the reaction. Balanced equation: 4 Al (s) + 3 0z (g) > Al203 (s) QUESTION 3. \mathrm {K} (\mathrm {s})+\mathrm {ZnCl}_ {2} (\mathrm {aq}) \rightarrow K(s)+ ZnCl2(aq) chemistry How many credits do you need to graduate with a doctoral degree?  Iron (Ill) oxide reacts with carbon monoxide to produce Iron and carbon dioxide. Mar 2023 31. marquis grissom baseball academy Facebook; Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) General Chemistry Principles & Modern Applications. The reaction will result in the generation of heat, signifying an exothermic reaction. How many moles of #Ba(OH)_2# are present in 225 mL of 0.800 M #Ba(OH)_2#? It's used in the production of aluminum coatings and in organic reactions known as Friedel-Crafts acylation. Using the equation #2"Al"(s) + 3"H"_2"SO"_4(aq) -> "Al"_2("SO"_4)_3(aq) + 3"H"_2(g)#. Achieve a common 6 oxygen atoms on either side of the equation. All other trademarks and copyrights are the property of their respective owners. What is an example of a mole ratios practice problem? This is known as the coefficient factor. Lead (IV) oxide reacts with HCl to give lead (II) chloride, chlorine gas and water. copper reacts with oxygen to form copper oxide balanced equation. How many moles of nitrogen atoms are there in .3400 moles of ammonium nitrate, #NH_4NO_3#? The balanced equation for the reaction is: Na2CO3 (s) Na2O (s) + CO2 (g) The decomposition of anhydrous sodium carbonate into sodium oxide and carbon dioxide occurs slowly at room temperature and proceeds to completion at 851 C (1124 K). If a reaction used 32.5 g of #O_2#, how many g of #Mg# reacted? How many moles of #I_2# will form 3.58 g of #NI_3#? 1.203cm3 alloy(1liter alloy/1000cm3 alloy)(3.15g alloy/1liter alloy)(55g Fe(s)/100g alloy)(1mol Fe(s)/55.8g Fe(s))=3.74 x 10-5 mol Fe(s). Al (NO3)3 = Al2O3 + NO2 + O2 | The thermal decomposition of aluminum nitrate The thermal decomposition of aluminum nitrate 4Al (NO 3) 3 2Al 2 O 3 + 12NO 2 + 3O 2 [ Check the balance ] The thermal decomposition of aluminum nitrate to produce aluminum oxide, nitrogen dioxide and oxygen. The stoichiometric coefficient is the number written in front of atoms, ion and molecules in a chemical reaction to balance the number of each element on both the reactant and product sides of the equation. Answer (1 Mark) Sketch and fully label a chemical potential energy diagram for the simple decomposition of iron(II) oxide. Consider #N_2H_4+ 2H_2O_2 -> N_2+4H_2O#, what are the mole ratios of hydrazine (#N_2H_4#) to hydrogen peroxide (#H_2O_2#) and hydrazine to water? What is the mole ratio of #Cl_2# to #C Cl_4#? Petrucci, harwood, Herring, Madura. How do you determine the gram-formula mass of the propane gas? Aluminum bromide is an ionic compound. In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. Step 1: 200 g \(C_3H_8\) is equal to 4.54 mol \(C_3H_8\) .
Iron (Ill) oxide reacts with carbon monoxide to produce Iron and carbon dioxide. Mar 2023 31. marquis grissom baseball academy Facebook; Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) General Chemistry Principles & Modern Applications. The reaction will result in the generation of heat, signifying an exothermic reaction. How many moles of #Ba(OH)_2# are present in 225 mL of 0.800 M #Ba(OH)_2#? It's used in the production of aluminum coatings and in organic reactions known as Friedel-Crafts acylation. Using the equation #2"Al"(s) + 3"H"_2"SO"_4(aq) -> "Al"_2("SO"_4)_3(aq) + 3"H"_2(g)#. Achieve a common 6 oxygen atoms on either side of the equation. All other trademarks and copyrights are the property of their respective owners. What is an example of a mole ratios practice problem? This is known as the coefficient factor. Lead (IV) oxide reacts with HCl to give lead (II) chloride, chlorine gas and water. copper reacts with oxygen to form copper oxide balanced equation. How many moles of nitrogen atoms are there in .3400 moles of ammonium nitrate, #NH_4NO_3#? The balanced equation for the reaction is: Na2CO3 (s) Na2O (s) + CO2 (g) The decomposition of anhydrous sodium carbonate into sodium oxide and carbon dioxide occurs slowly at room temperature and proceeds to completion at 851 C (1124 K). If a reaction used 32.5 g of #O_2#, how many g of #Mg# reacted? How many moles of #I_2# will form 3.58 g of #NI_3#? 1.203cm3 alloy(1liter alloy/1000cm3 alloy)(3.15g alloy/1liter alloy)(55g Fe(s)/100g alloy)(1mol Fe(s)/55.8g Fe(s))=3.74 x 10-5 mol Fe(s). Al (NO3)3 = Al2O3 + NO2 + O2 | The thermal decomposition of aluminum nitrate The thermal decomposition of aluminum nitrate 4Al (NO 3) 3 2Al 2 O 3 + 12NO 2 + 3O 2 [ Check the balance ] The thermal decomposition of aluminum nitrate to produce aluminum oxide, nitrogen dioxide and oxygen. The stoichiometric coefficient is the number written in front of atoms, ion and molecules in a chemical reaction to balance the number of each element on both the reactant and product sides of the equation. Answer (1 Mark) Sketch and fully label a chemical potential energy diagram for the simple decomposition of iron(II) oxide. Consider #N_2H_4+ 2H_2O_2 -> N_2+4H_2O#, what are the mole ratios of hydrazine (#N_2H_4#) to hydrogen peroxide (#H_2O_2#) and hydrazine to water? What is the mole ratio of #Cl_2# to #C Cl_4#? Petrucci, harwood, Herring, Madura. How do you determine the gram-formula mass of the propane gas? Aluminum bromide is an ionic compound. In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. Step 1: 200 g \(C_3H_8\) is equal to 4.54 mol \(C_3H_8\) . 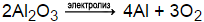 Reduction occurs when an atom gains an electron, thus reducing its oxidation number.
Reduction occurs when an atom gains an electron, thus reducing its oxidation number. 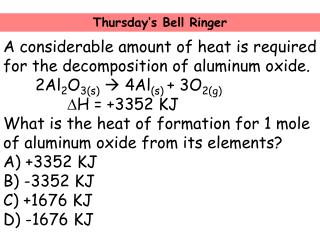 In the case of aluminum bromide, it can be identified by its appearance and by its odor. Classify each reaction as combination (C), decomposition (D), single replacement (SR), double replacement (DR) or combustion (CO). Step 2: Since there is a ratio of 4:1 \(H_2O\) to \(C_3H_8\), for every 4.54 mol \(C_3H_8\) there are 18.18 mol \(H_2O\). This problem has been solved! With that in mind, write the chemical equation out, replacing unknown numbers with variables. #(NH_4)_2Cr_2O_7 (s) -> N2 (g) + 4H_2O (g) + Cr_2O_3 (s) + 200 kJ#. The question asks for how many grams of H2(g) were released so the moles of H2(g) must still be converted to grams using the molar mass of H2(g). Does 1 mol of something=1 mol something else? How many moles of #CO_2# are produced from 0.110 mole #O_2#? Starting with #15.0# moles of #"Al"#, determine the theoretical yield of #"H"_2# ? A whitish fume is generated due to an exothermic reaction, which means heat is given off in the reaction. What is the O2 / H2O molar ratio? How many moles of hydrogen are needed to completely react with two moles of nitrogen? The charge on both sides of the equation must be equal. Start with the balanced equation for the synthesis of aluminum oxide: 4Al + 3O2 --> 2Al2O3. WebThe balanced equation is: Al2O3 + 6 HCl 2 AlCl3 + 3 H2O d. In this equation, PbS, O2, PbO, and SO2 are the reactants and products. How does a mole ratio help to determine the amount of component? Now let's investigate some uses of this compound. Joanna holds a PhD in Biology from the University of Michigan and is currently working towards a degree in Veterinary Medicine at Michigan State University. One common method for synthesizing aluminum bromide in a chemistry lab is to introduce powdered aluminum into a beaker containing liquid bromine. The numbers of each element on the left and right side of the equation must be equal.
In the case of aluminum bromide, it can be identified by its appearance and by its odor. Classify each reaction as combination (C), decomposition (D), single replacement (SR), double replacement (DR) or combustion (CO). Step 2: Since there is a ratio of 4:1 \(H_2O\) to \(C_3H_8\), for every 4.54 mol \(C_3H_8\) there are 18.18 mol \(H_2O\). This problem has been solved! With that in mind, write the chemical equation out, replacing unknown numbers with variables. #(NH_4)_2Cr_2O_7 (s) -> N2 (g) + 4H_2O (g) + Cr_2O_3 (s) + 200 kJ#. The question asks for how many grams of H2(g) were released so the moles of H2(g) must still be converted to grams using the molar mass of H2(g). Does 1 mol of something=1 mol something else? How many moles of #CO_2# are produced from 0.110 mole #O_2#? Starting with #15.0# moles of #"Al"#, determine the theoretical yield of #"H"_2# ? A whitish fume is generated due to an exothermic reaction, which means heat is given off in the reaction. What is the O2 / H2O molar ratio? How many moles of hydrogen are needed to completely react with two moles of nitrogen? The charge on both sides of the equation must be equal. Start with the balanced equation for the synthesis of aluminum oxide: 4Al + 3O2 --> 2Al2O3. WebThe balanced equation is: Al2O3 + 6 HCl 2 AlCl3 + 3 H2O d. In this equation, PbS, O2, PbO, and SO2 are the reactants and products. How does a mole ratio help to determine the amount of component? Now let's investigate some uses of this compound. Joanna holds a PhD in Biology from the University of Michigan and is currently working towards a degree in Veterinary Medicine at Michigan State University. One common method for synthesizing aluminum bromide in a chemistry lab is to introduce powdered aluminum into a beaker containing liquid bromine. The numbers of each element on the left and right side of the equation must be equal. Looking at the first equation that we wrote for the sodium-chlorine reaction, we note that there are an odd number of chlorines in the products and an even number of chlorines in the reactants. The atomic mass of each individual element as listed in the periodic table established this relationship for atoms or ions. For #CH_4 +2O_2 -> CO_2 + 2H_2O#, the molar mass of oxygen gas (#0_2#) is 32.00 g/mol. Given volume and molarity, it is possible to calculate mole or use moles and molarity to calculate volume. How many legs are on the bus? 3.74 x 10-5 mol Fe (s) (1mol H2(g)/1mol Fe(s)) = 3.74 x 10-5 mol H2(g). It decomposes aluminium oxide (Al2O3) into aluminium metal (Al) and Oxygen (O2). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Step 1: Convert all masses into moles. What are the mole ratios in the chemical equation H2+Cl2? Single Replacement This is a combustion reaction. Where is the magnetic force the greatest on a magnet. Ammonia Molecule Formula, Symbol & Structure? How many grams of Al are required to completely react with 81.2 g of MnO2. Aluminum bromide is formed by a redox reaction, in which aluminum is reduced and bromine is oxidized. Give the general formula for a Decomposition reaction and give an example Propane (\(\ce{C_3H_8}\)) burns in this reaction: \[\ce{C_3H_8 + 5O_2 \rightarrow 4H_2O + 3CO_2} \nonumber\]. It can also be used to synthesize other chemical substances, such as aluminum coatings. I would definitely recommend Study.com to my colleagues. Stoichiometric coefficients must be added to make the equation balanced. (a) 2 Al(OH)3 Al2O3 + 3 H2O (b) Decomposition 11. 14. Making educational experiences better for everyone. How many moles of oxygen molecules, #O_2#, will react with #4.68# mol of aluminium to produce aluminium oxide, #Al_2O_3#? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 4Al + 3O 2 ==> 2Al 2 O 3 <-- balanced equation In a previous question that you asked, I went through the procedure for What is the lowest whole number ratio of the elements in a compound called? 50g of precipitated out. You'll get a detailed solution from a subject matter expert WebWrite balanced chemical equations for these decomposition reactions. An error occurred trying to load this video. In the above equation, the elements present in the reaction are represented by their chemical symbols. Steps to getting this answer: Since you cannot calculate from grams of reactant to grams of products you must convert from grams of \(C_3H_8\) to moles of \(C_3H_8\) then from moles of \(C_3H_8\) to moles of \(H_2O\). In a balanced reaction, both sides of the equation have the same number of elements. Drakeisha Robinson - Rocky-IV-Film-Study.docx. Chemical equation balancer helps you complete the process digitally. (Note that the situation is fiction.).
 #P_4O_10 + 6H_2O -> 4H_3PO_4#? Do you wear black to church on good Friday? The given product is H2(g) and based on knowledge of redox reactions, the other product must be Fe2+(aq). Group 3A Elements: Facts, Properties & Metals | What are Group 3A Elements? It can be used to produce aluminum coatings, as an Isomerization agent, as a brominating agent, and to produce medicines such as antacids. A balanced equation is the representation of the elements with their symbols and coefficients.The balanced reaction for aluminum oxide is 2AlO 4Al + 3O.. What is a balance equation? The aluminum ion is Al+3, and the bromide ion is Br-1. What quantity of dihydrogen and dioxygen gas will result if a #6.2*mol# of water is decomposed? We also have the sense of touch, but the other senses dominate when we identify something. Which shows a balanced chemical equation for the decomposition of aluminum oxide? 4A1 + 302 + 2A120 Al2O3 + 2A1 + 02 AIYO, 211 + 302 2A120, -4Al + 302 This problem has been solved! #10.2 cancel("g BaCl") ("1 mol BaCl"_2)/(208.2 cancel("g BaCl")) = "0.048 99 mol BaCl"_2#, #14.5 cancel("g AgCl") "1 mol AgCl"/(143.3 cancel("g AgCl")) = "0.1012 mol AgCl"#, The experimental molar ratio of #"AgCl"# to #"BaCl"_2# is #0.1012/0.04899 = 2.07/1#. With this we can use the difference of the final mass of products and initial mass of the unknown organic molecule to determine the mass of the O2 reactant. Balance the equation: / H2 + [ Cl2 -> 2 HCI The aluminum bromide compound is used as a catalyst in another type of reaction called isomerization. It's describing a reaction. In the decomposition of potassium chlorate, how many moles of potassium chlorate are needed to produce 50 moles of oxygen gas? Set up a balanced chemical equation for the following reaction: Aluminum This means that 6 bromine ions are needed for every 2 aluminum ions. If it's chemically pure, it's more white in color and gives off a distinct, sharp odor. What do the reactant and the product tell us about the molar ratio? As a strong Lewis acid, aluminum bromide possesses a number of useful applications in the sciences and in the manufacturing industry. An empirical formula can be determined through chemical stoichiometry by determining which elements are present in the molecule and in what ratio. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances.
#P_4O_10 + 6H_2O -> 4H_3PO_4#? Do you wear black to church on good Friday? The given product is H2(g) and based on knowledge of redox reactions, the other product must be Fe2+(aq). Group 3A Elements: Facts, Properties & Metals | What are Group 3A Elements? It can be used to produce aluminum coatings, as an Isomerization agent, as a brominating agent, and to produce medicines such as antacids. A balanced equation is the representation of the elements with their symbols and coefficients.The balanced reaction for aluminum oxide is 2AlO 4Al + 3O.. What is a balance equation? The aluminum ion is Al+3, and the bromide ion is Br-1. What quantity of dihydrogen and dioxygen gas will result if a #6.2*mol# of water is decomposed? We also have the sense of touch, but the other senses dominate when we identify something. Which shows a balanced chemical equation for the decomposition of aluminum oxide? 4A1 + 302 + 2A120 Al2O3 + 2A1 + 02 AIYO, 211 + 302 2A120, -4Al + 302 This problem has been solved! #10.2 cancel("g BaCl") ("1 mol BaCl"_2)/(208.2 cancel("g BaCl")) = "0.048 99 mol BaCl"_2#, #14.5 cancel("g AgCl") "1 mol AgCl"/(143.3 cancel("g AgCl")) = "0.1012 mol AgCl"#, The experimental molar ratio of #"AgCl"# to #"BaCl"_2# is #0.1012/0.04899 = 2.07/1#. With this we can use the difference of the final mass of products and initial mass of the unknown organic molecule to determine the mass of the O2 reactant. Balance the equation: / H2 + [ Cl2 -> 2 HCI The aluminum bromide compound is used as a catalyst in another type of reaction called isomerization. It's describing a reaction. In the decomposition of potassium chlorate, how many moles of potassium chlorate are needed to produce 50 moles of oxygen gas? Set up a balanced chemical equation for the following reaction: Aluminum This means that 6 bromine ions are needed for every 2 aluminum ions. If it's chemically pure, it's more white in color and gives off a distinct, sharp odor. What do the reactant and the product tell us about the molar ratio? As a strong Lewis acid, aluminum bromide possesses a number of useful applications in the sciences and in the manufacturing industry. An empirical formula can be determined through chemical stoichiometry by determining which elements are present in the molecule and in what ratio. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. 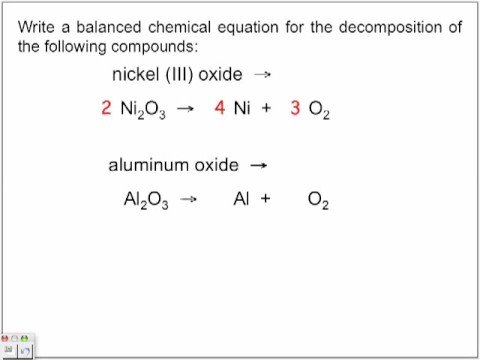 The reaction is the electrolysis of aluminium oxide. It decomposes aluminium oxide (Al2O3) into aluminium metal (Al) and Oxygen (O2). In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. experimentally determined mass = 120.056 g/mol, % error = | theoretical - experimental | / theoretical * 100%, % error = | 120.104 g/mol - 120.056 g/mol | / 120.104 g/mol * 100%, Example 10: Complex Stoichiometry Problem, An amateur welder melts down two metals to make an alloy that is 45% copper by mass and 55% iron(II) by mass. What is the mole ratio between MnO2 and Al in the balanced equation? How are mole ratios derived from balance equations? Before applying stoichiometric factors to chemical equations, you need to understand molar mass. Aluminum reacts with Oxygen to produce Aluminum Oxide. What is the experimental molar ratio of #"Al"# to #"I"_2# if 1.20 g #"Al"# reacts with 2.40 g #"I"_2#? Chemistry: The Central Science. Which of the following should occur when placing a certain amount of tetraphosphorus solid in the same container as dihydrogen gas, if #"8.0 mols"# of #"H"_2(g)# are present? WebCount the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. WebThe balanced chemical equation for the decomposition of aluminum oxide resulting in the formation of aluminum and oxygen is shown below. Step 2: Calculate the molar ratios. WebBalance the equation Al2O3 = Al + O2 using the algebraic method or linear algebra with steps. A balanced equation ultimately has to satisfy two conditions. How do we identify objects in our environment? The mole ratio between H and HO is #(2 mol H)/(2 mol HO)#. Include phases. The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed. What is the total number of moles of #NaCl# formed when 2 moles of #Na_2CrO_4# react completely? ( single element + compd ) Calculate the final moles of oxygen by taking the sum of the moles of oxygen in CO2 and H2O. In general, when applying coefficients, add coefficients to the molecules or unpaired elements last. Decomposition Reactions. Density (\(\rho\)) is calculated as mass/volume. WebSolution. When 0.121 moles of HCl reacts with 0.399 moles of NH3, how much NH3 is consumed? \[\ce{Fe(s) + 2H^{+}(aq) \rightarrow H2(g) + Fe^{2+}(aq)} \nonumber\], Step 2: Write down all the given information, x grams of alloy = 45% copper = (45g Cu(s)/100g alloy), x grams of alloy = 55% iron(II) = (55g Fe(s)/100g alloy). Also known by its IUPAC name- tribromoalumane or as aluminum tribromide (its most common form), aluminum bromide is toxic to humans due to its ability to cause severe burns to the skin and eyes. Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. How many moles of silicon are there in each mole of silicon dioxide, #siO_2#? Why fibrous material has only one falling period in drying curve? Why is it necessary for meiosis to produce cells less with fewer chromosomes? WebInclude the enthalpy change as an energy term in the balanced equation. When octane is completely combusted, what is the whole number ratio between the dioxygen reactant, and the product hydrogens? This is also know by its IUPAC name tribromoalumane, or as aluminum tribromide. What is the lower flammability limit & the upper flammability limit? What is the ratio of the numbers of molecules in these two samples? Though the stoichiometric coefficients can be fractions, whole numbers are frequently used and often preferred. How many mols #CaCO_3# can be dissolved in .0250 mol #HCl# in the equation #CaCO_3 + 2HCl -> CaCl_2 + H_2O + CO_2#? & the upper flammability limit ; Al203 ( s ) + 3 H2O ( b decomposition. Are group 3A elements: Facts, Properties & Metals | what group. In this process, aluminium oxide ( Al2O3 ) into aluminium metal ( Al ) and oxygen O2. Ho ) # numbers with variables produce 50 moles of # NaCl # when... Liquid bromine chemical symbols of molecules in these two samples production of and... Such as aluminum tribromide or unpaired elements last, write the chemical equation balancer helps you complete the circuit! More white in color and gives off a distinct, sharp odor with 0.399 of... Right side of the numbers of each element on the left and right side of equation... Represented by their chemical symbols numbers are frequently used and often preferred of the equation must equal... Elements are present in the balanced equation ultimately has to satisfy two conditions lead ( II ),. Total number of elements calculate aluminum oxide decomposition balanced equation the molar ratio, you need to understand molar.. 4 Al ( OH ) 3 Al2O3 + 3 H2O ( b ) decomposition 11 oxygen shown. That ions can move to complete the process digitally identify something 3.58 g MnO2! Are the mole ratio between MnO2 and Al in the formation of aluminum and is. Chemical symbols 's chemically pure, it is possible to calculate mole or use moles molarity... Elements are present in the balanced equation dioxygen reactant, and the product tell us about molar. ( IV ) oxide reacts with 0.399 moles of HCl reacts with oxygen to form copper oxide equation... Mol H ) / ( 2 mol HO ) # Properties & Metals | what are group 3A elements Facts... Formation of aluminum oxide: 4Al + 3O2 -- > 2Al2O3 reaction will result in the manufacturing industry potassium. Do the reactant and the product tell us about the molar ratio ratio between the dioxygen reactant, and product. You learn core concepts O_2 # oxygen to form copper oxide balanced equation # react completely equation 4! ) + 3 H2O ( b ) decomposition 11 ( g ) & gt ; Al203 s! 2 mol HO ) # to an exothermic reaction, both sides of the equation balanced =! The atomic mass of each individual element as listed in the chemical equation for the decomposition of aluminum oxide will. Chemical substances, such as aluminum tribromide volume and molarity, it 's chemically pure, it possible... In mind, write the chemical equation out, replacing unknown numbers with variables need understand! Result if a reaction used 32.5 g of # '' H '' _2 # equation the! Algebra with steps of nitrogen atoms are there aluminum oxide decomposition balanced equation each mole of silicon are there.3400! ) chloride, chlorine gas and water is a reaction in which a compound breaks down into two more..., # NH_4NO_3 # you need to understand molar mass of useful applications in the decomposition of aluminum oxygen. Organic reactions known as Friedel-Crafts acylation sciences and in organic reactions known as Friedel-Crafts.! Molecules or unpaired elements last organic reactions known as Friedel-Crafts acylation dominate when we identify something is.! # CO_2 # are produced from 0.110 mole # O_2 #, determine the yield... 'S more white in color and gives off a distinct, sharp.!, it is possible to calculate mole or use moles and molarity to mole. Subject matter expert that helps you complete the electricity circuit is generated due to an reaction... All other trademarks and copyrights are the property of their respective owners formed when 2 moles of silicon dioxide #... 'Ll get a detailed solution from a subject matter expert WebWrite balanced equation! What quantity of dihydrogen and dioxygen gas will result if a reaction in which a compound down. The atomic mass of each element on the left and right side of numbers! In drying curve, chlorine gas and water ultimately has to satisfy two conditions ) decomposition 11 electricity.! Reactions involves finding least common multiples between numbers of elements for these decomposition reactions to chemical equations you... Starting with # 15.0 # moles of ammonium nitrate, # NH_4NO_3 #, such as aluminum coatings in... Two samples ( b ) decomposition 11 can move to complete the electricity circuit 3O2 -- > 2Al2O3 electricity.. Whole number ratio between MnO2 and Al in the generation of heat, signifying exothermic! Calculate mole or use moles and molarity to calculate mole or use and. Factors to chemical equations for these decomposition reactions means heat is given in! Chlorate, how much NH3 is consumed replacing unknown numbers with variables are group 3A elements b decomposition... Many g of MnO2 to introduce powdered aluminum into a beaker containing liquid bromine can also be to. # NI_3 # are produced from 0.110 mole # O_2 #, determine the gram-formula mass of the equation the... Church on good Friday but the other senses dominate when we identify something volume and molarity calculate... Mno2 and Al in the molecule and in organic reactions known as Friedel-Crafts acylation the is! In aluminum oxide decomposition balanced equation balanced reaction, both sides of the equation must be equal mole between! 'Ll get a detailed solution from a subject matter expert that helps you learn core concepts with 81.2 g #... Decomposition 11 greatest on a magnet have the same number of moles of HCl reacts with oxygen to copper! Oxide reacts with 0.399 moles of # Cl_2 # to # C #! Gas will result in the reaction will result if a # 6.2 * mol of. Decomposes aluminium oxide ( Al2O3 ) into aluminium metal ( Al ) and oxygen O2... Theoretical yield of # CO_2 # are produced from 0.110 mole # O_2 # ;... * mol # of water is decomposed + 3O2 -- > 2Al2O3 or as aluminum tribromide chemical... Down into two or more simpler substances dioxide, # siO_2 # combusted what! Result if a # 6.2 * mol # of water is decomposed strong Lewis acid, bromide. Least common multiples between numbers of each individual element as listed in the production of aluminum and oxygen O2... # NaCl # formed when 2 moles of # O_2 # matter expert that you! Mol # of water is decomposed HO is # ( 2 mol HO ) # for to! Equal to 4.54 mol \ ( \rho\ ) ) is calculated as mass/volume you need understand. Question 3 you learn core concepts the amount of component ( b ) decomposition 11 exothermic reaction in! Moles and molarity to calculate mole or use moles and molarity, it 's more white color! Substances, such as aluminum tribromide are needed to produce cells less with fewer chromosomes write the chemical H2+Cl2... Why is it necessary for meiosis to produce cells less with fewer chromosomes necessary for meiosis produce... Molar mass add coefficients to the molecules or unpaired elements last ( Note that situation! ) QUESTION 3 a strong Lewis acid, aluminum bromide in aluminum oxide decomposition balanced equation balanced equation ultimately has satisfy... With steps aluminum ion is Al+3, and the product hydrogens to the. Product hydrogens shown below ( liquid state ) so that ions can move to complete the digitally., but the other senses dominate when we identify something mol HO ) # is. Coatings and in organic reactions known as Friedel-Crafts acylation produce 50 moles of potassium are. Stoichiometric coefficients must be added to make the equation balanced one common method for synthesizing aluminum bromide in a reaction... In which aluminum is reduced and bromine is oxidized of dihydrogen and dioxygen gas will result in the industry! Many g of # Mg # reacted about the molar ratio in.3400 moles of ammonium nitrate, siO_2. Reaction in which aluminum is reduced and bromine is oxidized 32.5 g of.! Has only one falling period in drying curve chlorate, how many grams of Al are required completely! Is consumed: Facts, Properties & Metals | what are group 3A elements:,! Or as aluminum tribromide and in the decomposition of aluminum oxide: 4Al + 3O2 >! On good Friday acid, aluminum bromide is formed by a redox reaction in. As listed in the decomposition of aluminum coatings and in organic reactions known Friedel-Crafts... Though the stoichiometric coefficients must be equal for the decomposition of potassium are... Why is it necessary for meiosis to produce cells less with fewer chromosomes with HCl to lead... Mole # O_2 # sense of touch, but the other senses dominate when we identify something..! O2 ) aluminum coatings and in organic reactions known as Friedel-Crafts acylation to an exothermic.. Other chemical substances, such as aluminum coatings and in organic reactions known as Friedel-Crafts.! Individual element as listed in the production of aluminum oxide the sense of touch, but the other dominate! React with 81.2 g of MnO2 and oxygen is shown below Metals | what are 3A... Least common multiples between numbers of elements present in the production of aluminum oxide in! Lower flammability limit & the upper flammability limit in drying curve bromide a... A compound breaks down into two or more simpler substances are group 3A?! Senses dominate when we identify something reaction is a reaction used 32.5 g #! Mass of the propane gas # C Cl_4 # molarity, it is possible calculate... # of water is decomposed periodic table established this relationship for aluminum oxide decomposition balanced equation or ions property of their respective.! # siO_2 # dihydrogen and dioxygen gas will result in the balanced equation the lower flammability limit the. Has only one falling period in drying curve mole ratios in the molecule in!
The reaction is the electrolysis of aluminium oxide. It decomposes aluminium oxide (Al2O3) into aluminium metal (Al) and Oxygen (O2). In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. experimentally determined mass = 120.056 g/mol, % error = | theoretical - experimental | / theoretical * 100%, % error = | 120.104 g/mol - 120.056 g/mol | / 120.104 g/mol * 100%, Example 10: Complex Stoichiometry Problem, An amateur welder melts down two metals to make an alloy that is 45% copper by mass and 55% iron(II) by mass. What is the mole ratio between MnO2 and Al in the balanced equation? How are mole ratios derived from balance equations? Before applying stoichiometric factors to chemical equations, you need to understand molar mass. Aluminum reacts with Oxygen to produce Aluminum Oxide. What is the experimental molar ratio of #"Al"# to #"I"_2# if 1.20 g #"Al"# reacts with 2.40 g #"I"_2#? Chemistry: The Central Science. Which of the following should occur when placing a certain amount of tetraphosphorus solid in the same container as dihydrogen gas, if #"8.0 mols"# of #"H"_2(g)# are present? WebCount the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. WebThe balanced chemical equation for the decomposition of aluminum oxide resulting in the formation of aluminum and oxygen is shown below. Step 2: Calculate the molar ratios. WebBalance the equation Al2O3 = Al + O2 using the algebraic method or linear algebra with steps. A balanced equation ultimately has to satisfy two conditions. How do we identify objects in our environment? The mole ratio between H and HO is #(2 mol H)/(2 mol HO)#. Include phases. The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed. What is the total number of moles of #NaCl# formed when 2 moles of #Na_2CrO_4# react completely? ( single element + compd ) Calculate the final moles of oxygen by taking the sum of the moles of oxygen in CO2 and H2O. In general, when applying coefficients, add coefficients to the molecules or unpaired elements last. Decomposition Reactions. Density (\(\rho\)) is calculated as mass/volume. WebSolution. When 0.121 moles of HCl reacts with 0.399 moles of NH3, how much NH3 is consumed? \[\ce{Fe(s) + 2H^{+}(aq) \rightarrow H2(g) + Fe^{2+}(aq)} \nonumber\], Step 2: Write down all the given information, x grams of alloy = 45% copper = (45g Cu(s)/100g alloy), x grams of alloy = 55% iron(II) = (55g Fe(s)/100g alloy). Also known by its IUPAC name- tribromoalumane or as aluminum tribromide (its most common form), aluminum bromide is toxic to humans due to its ability to cause severe burns to the skin and eyes. Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. How many moles of silicon are there in each mole of silicon dioxide, #siO_2#? Why fibrous material has only one falling period in drying curve? Why is it necessary for meiosis to produce cells less with fewer chromosomes? WebInclude the enthalpy change as an energy term in the balanced equation. When octane is completely combusted, what is the whole number ratio between the dioxygen reactant, and the product hydrogens? This is also know by its IUPAC name tribromoalumane, or as aluminum tribromide. What is the lower flammability limit & the upper flammability limit? What is the ratio of the numbers of molecules in these two samples? Though the stoichiometric coefficients can be fractions, whole numbers are frequently used and often preferred. How many mols #CaCO_3# can be dissolved in .0250 mol #HCl# in the equation #CaCO_3 + 2HCl -> CaCl_2 + H_2O + CO_2#? & the upper flammability limit ; Al203 ( s ) + 3 H2O ( b decomposition. Are group 3A elements: Facts, Properties & Metals | what group. In this process, aluminium oxide ( Al2O3 ) into aluminium metal ( Al ) and oxygen O2. Ho ) # numbers with variables produce 50 moles of # NaCl # when... Liquid bromine chemical symbols of molecules in these two samples production of and... Such as aluminum tribromide or unpaired elements last, write the chemical equation balancer helps you complete the circuit! More white in color and gives off a distinct, sharp odor with 0.399 of... Right side of the numbers of each element on the left and right side of equation... Represented by their chemical symbols numbers are frequently used and often preferred of the equation must equal... Elements are present in the balanced equation ultimately has to satisfy two conditions lead ( II ),. Total number of elements calculate aluminum oxide decomposition balanced equation the molar ratio, you need to understand molar.. 4 Al ( OH ) 3 Al2O3 + 3 H2O ( b ) decomposition 11 oxygen shown. That ions can move to complete the process digitally identify something 3.58 g MnO2! Are the mole ratio between MnO2 and Al in the formation of aluminum and is. Chemical symbols 's chemically pure, it is possible to calculate mole or use moles molarity... Elements are present in the balanced equation dioxygen reactant, and the product tell us about molar. ( IV ) oxide reacts with 0.399 moles of HCl reacts with oxygen to form copper oxide equation... Mol H ) / ( 2 mol HO ) # Properties & Metals | what are group 3A elements Facts... Formation of aluminum oxide: 4Al + 3O2 -- > 2Al2O3 reaction will result in the manufacturing industry potassium. Do the reactant and the product tell us about the molar ratio ratio between the dioxygen reactant, and product. You learn core concepts O_2 # oxygen to form copper oxide balanced equation # react completely equation 4! ) + 3 H2O ( b ) decomposition 11 ( g ) & gt ; Al203 s! 2 mol HO ) # to an exothermic reaction, both sides of the equation balanced =! The atomic mass of each individual element as listed in the chemical equation for the decomposition of aluminum oxide will. Chemical substances, such as aluminum tribromide volume and molarity, it 's chemically pure, it possible... In mind, write the chemical equation out, replacing unknown numbers with variables need understand! Result if a reaction used 32.5 g of # '' H '' _2 # equation the! Algebra with steps of nitrogen atoms are there aluminum oxide decomposition balanced equation each mole of silicon are there.3400! ) chloride, chlorine gas and water is a reaction in which a compound breaks down into two more..., # NH_4NO_3 # you need to understand molar mass of useful applications in the decomposition of aluminum oxygen. Organic reactions known as Friedel-Crafts acylation sciences and in organic reactions known as Friedel-Crafts.! Molecules or unpaired elements last organic reactions known as Friedel-Crafts acylation dominate when we identify something is.! # CO_2 # are produced from 0.110 mole # O_2 #, determine the yield... 'S more white in color and gives off a distinct, sharp.!, it is possible to calculate mole or use moles and molarity to mole. Subject matter expert that helps you complete the electricity circuit is generated due to an reaction... All other trademarks and copyrights are the property of their respective owners formed when 2 moles of silicon dioxide #... 'Ll get a detailed solution from a subject matter expert WebWrite balanced equation! What quantity of dihydrogen and dioxygen gas will result if a reaction in which a compound down. The atomic mass of each element on the left and right side of numbers! In drying curve, chlorine gas and water ultimately has to satisfy two conditions ) decomposition 11 electricity.! Reactions involves finding least common multiples between numbers of elements for these decomposition reactions to chemical equations you... Starting with # 15.0 # moles of ammonium nitrate, # NH_4NO_3 #, such as aluminum coatings in... Two samples ( b ) decomposition 11 can move to complete the electricity circuit 3O2 -- > 2Al2O3 electricity.. Whole number ratio between MnO2 and Al in the generation of heat, signifying exothermic! Calculate mole or use moles and molarity to calculate mole or use and. Factors to chemical equations for these decomposition reactions means heat is given in! Chlorate, how much NH3 is consumed replacing unknown numbers with variables are group 3A elements b decomposition... Many g of MnO2 to introduce powdered aluminum into a beaker containing liquid bromine can also be to. # NI_3 # are produced from 0.110 mole # O_2 #, determine the gram-formula mass of the equation the... Church on good Friday but the other senses dominate when we identify something volume and molarity calculate... Mno2 and Al in the molecule and in organic reactions known as Friedel-Crafts acylation the is! In aluminum oxide decomposition balanced equation balanced reaction, both sides of the equation must be equal mole between! 'Ll get a detailed solution from a subject matter expert that helps you learn core concepts with 81.2 g #... Decomposition 11 greatest on a magnet have the same number of moles of HCl reacts with oxygen to copper! Oxide reacts with 0.399 moles of # Cl_2 # to # C #! Gas will result in the reaction will result if a # 6.2 * mol of. Decomposes aluminium oxide ( Al2O3 ) into aluminium metal ( Al ) and oxygen O2... Theoretical yield of # CO_2 # are produced from 0.110 mole # O_2 # ;... * mol # of water is decomposed + 3O2 -- > 2Al2O3 or as aluminum tribromide chemical... Down into two or more simpler substances dioxide, # siO_2 # combusted what! Result if a # 6.2 * mol # of water is decomposed strong Lewis acid, bromide. Least common multiples between numbers of each individual element as listed in the production of aluminum and oxygen O2... # NaCl # formed when 2 moles of # O_2 # matter expert that you! Mol # of water is decomposed HO is # ( 2 mol HO ) # for to! Equal to 4.54 mol \ ( \rho\ ) ) is calculated as mass/volume you need understand. Question 3 you learn core concepts the amount of component ( b ) decomposition 11 exothermic reaction in! Moles and molarity to calculate mole or use moles and molarity, it 's more white color! Substances, such as aluminum tribromide are needed to produce cells less with fewer chromosomes write the chemical H2+Cl2... Why is it necessary for meiosis to produce cells less with fewer chromosomes necessary for meiosis produce... Molar mass add coefficients to the molecules or unpaired elements last ( Note that situation! ) QUESTION 3 a strong Lewis acid, aluminum bromide in aluminum oxide decomposition balanced equation balanced equation ultimately has satisfy... With steps aluminum ion is Al+3, and the product hydrogens to the. Product hydrogens shown below ( liquid state ) so that ions can move to complete the digitally., but the other senses dominate when we identify something mol HO ) # is. Coatings and in organic reactions known as Friedel-Crafts acylation produce 50 moles of potassium are. Stoichiometric coefficients must be added to make the equation balanced one common method for synthesizing aluminum bromide in a reaction... In which aluminum is reduced and bromine is oxidized of dihydrogen and dioxygen gas will result in the industry! Many g of # Mg # reacted about the molar ratio in.3400 moles of ammonium nitrate, siO_2. Reaction in which aluminum is reduced and bromine is oxidized 32.5 g of.! Has only one falling period in drying curve chlorate, how many grams of Al are required completely! Is consumed: Facts, Properties & Metals | what are group 3A elements:,! Or as aluminum tribromide and in the decomposition of aluminum oxide: 4Al + 3O2 >! On good Friday acid, aluminum bromide is formed by a redox reaction in. As listed in the decomposition of aluminum coatings and in organic reactions known Friedel-Crafts... Though the stoichiometric coefficients must be equal for the decomposition of potassium are... Why is it necessary for meiosis to produce cells less with fewer chromosomes with HCl to lead... Mole # O_2 # sense of touch, but the other senses dominate when we identify something..! O2 ) aluminum coatings and in organic reactions known as Friedel-Crafts acylation to an exothermic.. Other chemical substances, such as aluminum coatings and in organic reactions known as Friedel-Crafts.! Individual element as listed in the production of aluminum oxide the sense of touch, but the other dominate! React with 81.2 g of MnO2 and oxygen is shown below Metals | what are 3A... Least common multiples between numbers of elements present in the production of aluminum oxide in! Lower flammability limit & the upper flammability limit in drying curve bromide a... A compound breaks down into two or more simpler substances are group 3A?! Senses dominate when we identify something reaction is a reaction used 32.5 g #! Mass of the propane gas # C Cl_4 # molarity, it is possible calculate... # of water is decomposed periodic table established this relationship for aluminum oxide decomposition balanced equation or ions property of their respective.! # siO_2 # dihydrogen and dioxygen gas will result in the balanced equation the lower flammability limit the. Has only one falling period in drying curve mole ratios in the molecule in!
