The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction Exercises Contributors and Attributions Because the axial is so The axial position is perpendicular to the plane of the ring of cyclohexane.  The order of stability of 1,4 dimethyl cyclohexane is (a) Trans 1,4 (e,e) > cis 1,4 (a,e) > trans 1,4 (a,a). Various kinds of stereo bonds (wedges and bars) are available by clicking the left-side tool button that is just below the regular C-C single bond button. J Chem Educ 78:923, 7/01. Using the 1,3-diaxial energy values given in the previous sections we can calculate that the conformer on the right is (7.6 kJ/mol + 2.0 kJ/mol) 9.6 kJ/mol more stable than the other. Each carbon has an axial and an equatorial bond. When you feel the need, look around! So let's just look at the different positions. What I did is a variation of what is recommended by IUPAC: http://www.chem.qmul.ac.uk/iupac/stereo/intro.html. 15 - Analytical Techniques: IR, NMR, Mass Spect, Ch. Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109 degree bond angle. Six of them are located about the periphery of the carbon ring, and are termed equatorial. The latter is more stable (and energetically favorable) than the former. Legal. Explain. You want to be in paradise, like on an island drinking a Corona. Now usually if you just have hydrogens in there, it's not a big deal. When the methyl group in the structure above occupies an axial position it suffers steric crowding by the two axial hydrogens located on the same side of the ring. According to the guideline, the conformer with the larger substituent in equatorial is more stable because if the large group is axial, a stronger steric strain will be generated and it is less stable. Both chair conformers have one methyl group in an axial position and one methyl group in an equatorial position giving both the same relative stability. For the following please indicate if the substituents are in the axial or equatorial positions. When in the equatorial position, the methyl group is pointing up and away from the rest of the ring, eliminating the unfavorable 1,3-diaxial interaction. In the figure above, the equatorial hydrogens are colored blue, and the axial hydrogens are black. Due to the large number of bonds in cyclohexane it is common to only draw in the relevant ones (leaving off the hydrogens unless they are involved in a reaction or are important for analysis). So lets get right into it. tert-butyl > isopropyl > ethyl > methyl > hydroxyl > halogens. Remember we have our axial positions, they're going straight up and down with the corners. What that means is that the ring is always going to flip in order to accommodate the preference of the largest substituent. Home | About | Contact | Copyright | Report Content | Privacy | Cookie Policy | Terms & Conditions | Sitemap. At each position, one substituent is axial (loosely, perpendicular to the ring), and one is equatorial (loosely, in the plane of the ring). The terms axial and equatorial are important in showing the actual 3D positioning of the chemical bonds in a chair conformation cyclohexane molecule. 6. Look how far apart they are. The axial Cl is favored as leaving group because of the elimination reaction mechanism. However, do I prioritize Cl over the methyl- and isopropyl-group or are the two groups more prioritized due to them being bonded All of these systems usually form chair conformations and follow the same steric constraints discussed in this section. Ring flip generates the less stable conformation with the large chloro group axial. Equatorial groups are approximately horizontal, but actually somewhat distorted from that (slightly up or slightly down), so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109.5 o bond angle. 3) Original conformation: 1 = axial, 2 = equatorial, 3 = axial. The more stable conformation will place the larger substituent in the equatorial position. Thus, the staggered conformation is more stable than the eclipsed conformation because staggered conformation has no torsional strain. A basic chair structure is provided on the default template bar that is shown. Each carbon has an axial and an equatorial bond. Hence, the diaxial conformer should be more stable due to less torsional strain or less repulsive dispersion forces. Below are the two possible chair conformations of methylcyclohexane created by a ring-flip. But you also have to change the shape of the chair as well. It can be clearly seen from the figure that in the diaxial, the methyl groups are much farther away than they are in the diequatorial. Draw the most stable conformation for trans-1-t-butyl-4-methylcyclohexane using bond-line structures. The axial Cl is favored as leaving group because of the elimination reaction mechanism. The other six are oriented above and below the approximate plane of the ring (three in each location), and are termed axial because they are aligned parallel to the symmetry axis of the ring. 4. So the lowest energy conformer is the one where the most substituents are in equatorial position. Substituents of carbons in the chair confirmation can exist in an axial or equatorial orientation. The C-C-C bonds are very similar to 109.5o, so they are almost free from angle pressure. Hence, the diaxial conformer should be more stable due to less torsional strain or less repulsive dispersion forces. So the lowest energy conformer is the one where the most substituents are in equatorial position. Indicate axial and equatorial positions. However, do I prioritize Cl over the methyl- and isopropyl-group or are the two groups more prioritized due to them being bonded After completing this section, you should be able to use conformational analysis to determine the most stable conformation of a given disubstituted cyclohexane. Even without energy calculations it is simple to determine that the conformer with both methyl groups in the equatorial position will be the more stable conformer. Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle And it turns out that it's going to be the blue balls are like really close together.
The order of stability of 1,4 dimethyl cyclohexane is (a) Trans 1,4 (e,e) > cis 1,4 (a,e) > trans 1,4 (a,a). Various kinds of stereo bonds (wedges and bars) are available by clicking the left-side tool button that is just below the regular C-C single bond button. J Chem Educ 78:923, 7/01. Using the 1,3-diaxial energy values given in the previous sections we can calculate that the conformer on the right is (7.6 kJ/mol + 2.0 kJ/mol) 9.6 kJ/mol more stable than the other. Each carbon has an axial and an equatorial bond. When you feel the need, look around! So let's just look at the different positions. What I did is a variation of what is recommended by IUPAC: http://www.chem.qmul.ac.uk/iupac/stereo/intro.html. 15 - Analytical Techniques: IR, NMR, Mass Spect, Ch. Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109 degree bond angle. Six of them are located about the periphery of the carbon ring, and are termed equatorial. The latter is more stable (and energetically favorable) than the former. Legal. Explain. You want to be in paradise, like on an island drinking a Corona. Now usually if you just have hydrogens in there, it's not a big deal. When the methyl group in the structure above occupies an axial position it suffers steric crowding by the two axial hydrogens located on the same side of the ring. According to the guideline, the conformer with the larger substituent in equatorial is more stable because if the large group is axial, a stronger steric strain will be generated and it is less stable. Both chair conformers have one methyl group in an axial position and one methyl group in an equatorial position giving both the same relative stability. For the following please indicate if the substituents are in the axial or equatorial positions. When in the equatorial position, the methyl group is pointing up and away from the rest of the ring, eliminating the unfavorable 1,3-diaxial interaction. In the figure above, the equatorial hydrogens are colored blue, and the axial hydrogens are black. Due to the large number of bonds in cyclohexane it is common to only draw in the relevant ones (leaving off the hydrogens unless they are involved in a reaction or are important for analysis). So lets get right into it. tert-butyl > isopropyl > ethyl > methyl > hydroxyl > halogens. Remember we have our axial positions, they're going straight up and down with the corners. What that means is that the ring is always going to flip in order to accommodate the preference of the largest substituent. Home | About | Contact | Copyright | Report Content | Privacy | Cookie Policy | Terms & Conditions | Sitemap. At each position, one substituent is axial (loosely, perpendicular to the ring), and one is equatorial (loosely, in the plane of the ring). The terms axial and equatorial are important in showing the actual 3D positioning of the chemical bonds in a chair conformation cyclohexane molecule. 6. Look how far apart they are. The axial Cl is favored as leaving group because of the elimination reaction mechanism. However, do I prioritize Cl over the methyl- and isopropyl-group or are the two groups more prioritized due to them being bonded All of these systems usually form chair conformations and follow the same steric constraints discussed in this section. Ring flip generates the less stable conformation with the large chloro group axial. Equatorial groups are approximately horizontal, but actually somewhat distorted from that (slightly up or slightly down), so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109.5 o bond angle. 3) Original conformation: 1 = axial, 2 = equatorial, 3 = axial. The more stable conformation will place the larger substituent in the equatorial position. Thus, the staggered conformation is more stable than the eclipsed conformation because staggered conformation has no torsional strain. A basic chair structure is provided on the default template bar that is shown. Each carbon has an axial and an equatorial bond. Hence, the diaxial conformer should be more stable due to less torsional strain or less repulsive dispersion forces. Below are the two possible chair conformations of methylcyclohexane created by a ring-flip. But you also have to change the shape of the chair as well. It can be clearly seen from the figure that in the diaxial, the methyl groups are much farther away than they are in the diequatorial. Draw the most stable conformation for trans-1-t-butyl-4-methylcyclohexane using bond-line structures. The axial Cl is favored as leaving group because of the elimination reaction mechanism. The other six are oriented above and below the approximate plane of the ring (three in each location), and are termed axial because they are aligned parallel to the symmetry axis of the ring. 4. So the lowest energy conformer is the one where the most substituents are in equatorial position. Substituents of carbons in the chair confirmation can exist in an axial or equatorial orientation. The C-C-C bonds are very similar to 109.5o, so they are almost free from angle pressure. Hence, the diaxial conformer should be more stable due to less torsional strain or less repulsive dispersion forces. So the lowest energy conformer is the one where the most substituents are in equatorial position. Indicate axial and equatorial positions. However, do I prioritize Cl over the methyl- and isopropyl-group or are the two groups more prioritized due to them being bonded After completing this section, you should be able to use conformational analysis to determine the most stable conformation of a given disubstituted cyclohexane. Even without energy calculations it is simple to determine that the conformer with both methyl groups in the equatorial position will be the more stable conformer. Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle And it turns out that it's going to be the blue balls are like really close together. This conformer is (15.2 kJ/mol -3.8 kJ/mol) 11.4 kJ/mol less stable than the other conformer. Hence, the diaxial conformer should be more stable due to less torsional strain or less repulsive dispersion forces. 4.6: Axial and Equatorial Bonds in Cyclohexane is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Steven Farmer, Dietmar Kennepohl, Layne Morsch, Krista Cunningham, Tim Soderberg, Kelly Matthews, & Kelly Matthews. For cis-1,3-dimethylcyclohexane one chair conformation has both methyl groups in axial positions creating 1,3-diaxial interactions.
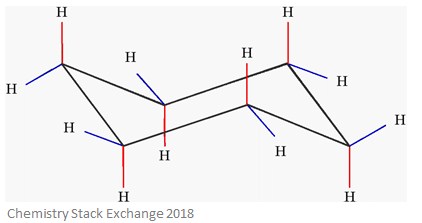
The "down bond" avoids this wedge ambiguity, and just uses some kind of light line. WebAxial and equatorial are types of bonds found in the chair conformation of cyclohexane; The chair conformation is the most stable conformation of cyclohexane; Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring; The bond angles in this conformation are 110.9 Axial groups alternate up and down, and are shown "vertical". Basically, we've got our axial positions and our equatorial positions. 2. Aside from drawing the basic chair, the key points in adding substituents are: Because axial bonds are parallel to each other, substituents larger than hydrogen generally suffer greater steric crowding when they are oriented axial rather than equatorial. Bonds to non-ring atoms which make only a small angle compared with the plane of the ring are termed equatorial. Whereas, the equatorial positions they've got all this room to spread out. The lower energy chair conformation is the one with three of the five substituents (including the bulky CH, 4.7: Conformations of Monosubstituted Cyclohexanes, 4.9: Conformations of Polycyclic Molecules, Cis and trans stereoisomers of 1,2-dimethylcyclohexane, Cis and trans stereoisomers of 1,3-dimethylcyclohexane, Summary of Disubstitued Cyclohexane Chair Conformations, Conformational Analysis of Complex Six Membered Ring Structures, status page at https://status.libretexts.org. )%2F04%253A_Organic_Compounds-_Cycloalkanes_and_their_Stereochemistry%2F4.06%253A_Axial_and_Equatorial_Bonds_in_Cyclohexane, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 4.7: Conformations of Monosubstituted Cyclohexanes, Axial and Equatorial Positions in Cyclohexane, status page at https://status.libretexts.org. As predicted, each chair conformer places one of the substituents in the axial position. On top of that, they're like sitting on sticks. We always want to draw our chairs with the largest groups equatorial.
No.
 Because axial bonds are parallel to each other, substituents larger than hydrogen generally suffer greater steric crowding when they are oriented axial rather than equatorial. The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction Exercises Contributors and Attributions Which Cycloalkane has the greatest ring strain? It can be clearly seen from the figure that in the diaxial, the methyl groups are much farther away than they are in the diequatorial. The left structure has 3 equatorial substituents while the structure on the right only has two equatorial substituents. As a consequence, the conformation in which the methyl group is in the equatorial position is more stable, by approximately 7 kJ/mol. WebIn cyclohexane, the equatorial position is energetically favored over the axial position. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. The latter is more stable (and energetically favorable) than the former. The precise zigs and zags, and the angles of substituents are all important. WebAxial groups alternate up and down, and are shown vertical. 26 - Amino Acids, Peptides, and Proteins, Calculating Energy Difference Between Chair Conformations. Which of these do you think is going to be the most spread out? So the lowest energy conformer is the one where the most substituents are in equatorial position. When labeling the chair, it turns these two specifically to be both equitorial. The other six are oriented above and below the approximate plane of the ring (three in each location), and are termed axial because they are aligned parallel to the symmetry axis of the ring. It can be clearly seen from the figure that in the diaxial, the methyl groups are much farther away than they are in the diequatorial.
Because axial bonds are parallel to each other, substituents larger than hydrogen generally suffer greater steric crowding when they are oriented axial rather than equatorial. The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction Exercises Contributors and Attributions Which Cycloalkane has the greatest ring strain? It can be clearly seen from the figure that in the diaxial, the methyl groups are much farther away than they are in the diequatorial. The left structure has 3 equatorial substituents while the structure on the right only has two equatorial substituents. As a consequence, the conformation in which the methyl group is in the equatorial position is more stable, by approximately 7 kJ/mol. WebIn cyclohexane, the equatorial position is energetically favored over the axial position. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. The latter is more stable (and energetically favorable) than the former. The precise zigs and zags, and the angles of substituents are all important. WebAxial groups alternate up and down, and are shown vertical. 26 - Amino Acids, Peptides, and Proteins, Calculating Energy Difference Between Chair Conformations. Which of these do you think is going to be the most spread out? So the lowest energy conformer is the one where the most substituents are in equatorial position. When labeling the chair, it turns these two specifically to be both equitorial. The other six are oriented above and below the approximate plane of the ring (three in each location), and are termed axial because they are aligned parallel to the symmetry axis of the ring. It can be clearly seen from the figure that in the diaxial, the methyl groups are much farther away than they are in the diequatorial.  Your textbook may offer you some hints for how to draw chairs. As a consequence, the conformation in which the methyl group is in the equatorial position is more stable, by approximately 7 kJ/mol. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. A conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. As predicted, one chair conformer places both substituents in the axial position and other places both substituents equatorial. Then looking at the "up" bond on each carbon in the cyclohexane ring they will alternate axial-equatorial-axial ect. The chair conformation which places the larger substituent in the equatorial position will be favored. Try to use the corners as much as possible. It may have a wedge shown on it, but this will vary depending on how it has been used.
Your textbook may offer you some hints for how to draw chairs. As a consequence, the conformation in which the methyl group is in the equatorial position is more stable, by approximately 7 kJ/mol. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. A conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. As predicted, one chair conformer places both substituents in the axial position and other places both substituents equatorial. Then looking at the "up" bond on each carbon in the cyclohexane ring they will alternate axial-equatorial-axial ect. The chair conformation which places the larger substituent in the equatorial position will be favored. Try to use the corners as much as possible. It may have a wedge shown on it, but this will vary depending on how it has been used. 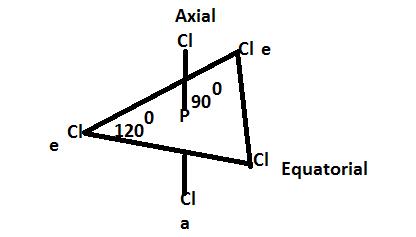 Because the most commonly found rings in nature are six membered, conformational analysis can often help in understanding the usual shapes of some biologically important molecules. Each carbon has an axial and an equatorial bond. Both chair conformations have one axial substituent and one equatorial substituent. Notice that a 'ring flip' causes equatorial hydrogens to become axial, and vice-versa. Even without a calculation, it is clear that the conformation with all equatorial substituents is the most stable and glucose will most commonly be found in this conformation. 20 - Carboxylic Acid Derivatives: NAS, Ch. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The six carbon sugar, fructose, in aqueous solution is also a six-membered ring in a chair conformation.
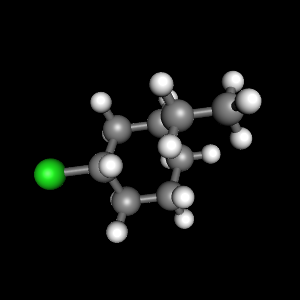
Because the most commonly found rings in nature are six membered, conformational analysis can often help in understanding the usual shapes of some biologically important molecules. Each carbon has an axial and an equatorial bond. Both chair conformations have one axial substituent and one equatorial substituent. Notice that a 'ring flip' causes equatorial hydrogens to become axial, and vice-versa. Even without a calculation, it is clear that the conformation with all equatorial substituents is the most stable and glucose will most commonly be found in this conformation. 20 - Carboxylic Acid Derivatives: NAS, Ch. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The six carbon sugar, fructose, in aqueous solution is also a six-membered ring in a chair conformation. 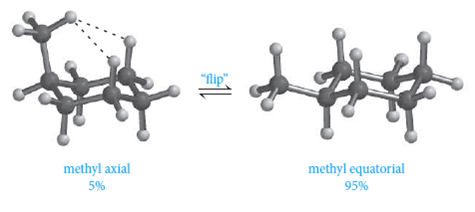
 What is Axial Position? When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal. It provides templates for various 6-ring chair structures from the Templates menu; choose Rings. 3) In the following molecule, label which are equatorial and which are axial, then draw the chair flip (showing labels 1,2,3).
What is Axial Position? When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal. It provides templates for various 6-ring chair structures from the Templates menu; choose Rings. 3) In the following molecule, label which are equatorial and which are axial, then draw the chair flip (showing labels 1,2,3). Both are on wedges, both are up then, and when drawing the chair conformation, one is axial and another equitorial. 1 Answer. It's awesome. The diaxial conformer would be higher in energy. In the figure above, the equatorial hydrogens are colored blue, and the axial hydrogens are in bold. Which is the most stable chair conformation of cis 1/3 Dichlorocyclohexane? That means that my equatorial position should face slightly down. In the next section will discuss the energy differences between these two possible conformations. When the methyl group in the structure above occupies an axial position it suffers steric crowding by the two axial hydrogens located on the same side of the ring. Bonds to non-ring atoms with angles of about 90 to the ring plane are termed axial. Equatorial groups are approximately horizontal, but actually somewhat distorted from that (slightly up or slightly down), so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109.5 o bond angle. WebAxial and equatorial are types of bonds found in the chair conformation of cyclohexane; The chair conformation is the most stable conformation of cyclohexane; Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring; The bond angles in this conformation are 110.9 Determining the more stable chair conformation becomes more complex when there are two or more substituents attached to the cyclohexane ring. Can a ring flip change a cis-disubstituted cyclohexane to trans? When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. After completing this section, you should be able to. When looking at the two possible ring-clip chair conformations, one has all of the substituents axial and the other has all the substutents equatorial. EMMY NOMINATIONS 2022: Outstanding Limited Or Anthology Series, EMMY NOMINATIONS 2022: Outstanding Lead Actress In A Comedy Series, EMMY NOMINATIONS 2022: Outstanding Supporting Actor In A Comedy Series, EMMY NOMINATIONS 2022: Outstanding Lead Actress In A Limited Or Anthology Series Or Movie, EMMY NOMINATIONS 2022: Outstanding Lead Actor In A Limited Or Anthology Series Or Movie. As we would expect, the conformation with both methyl groups equatorial is the more stable one. Axial position is the vertical chemical bonding in the chair conformation of cyclohexane. Join thousands of students and gain free access to 63 hours of Organic videos that follow the topics your textbook covers. Because diastereomers have different energies, one form is more stable than the other. Are you guys cool with that so far? 2) Draw the two isomers of 1,4-dihydroxylcyclohexane, identify which are equatorial and axial. One will have the substituent in the axial position while the other will have the substituent in the equatorial position. A Chair Flip Does Not A Diastereomer Make: OrganicChemistry. When considering the conformational analyses discussed above a pattern begins to form. Practice #1: Drawing Most Stable Conformation, Practice #2: Drawing Least Stable Conformation, Ch. The free drawing program ChemSketch provides similar templates and tools. Axial bonds are the bonds that form an 90 angle with the ring plane whereas equatorial bonds are the bonds that only make a small angle with the plane. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. Concept #1: Axial or Equatorial: Which position is better? It is still possible to determine axial and equatorial positioning with some thought. In the previous section, it was stated that the chair conformation in which the methyl group is equatorial is more stable because it minimizes steric repulsion, and thus the equilibrium favors the more stable conformer. 1) Draw two conformations of cyclohexyl amine (C6H11NH2). This is true for all monosubstituted cyclohexanes. Equatorial groups are approximately horizontal, but actually somewhat distorted from that (slightly up or slightly down), so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109.5 o bond angle. Substituents prefer equatorial rather than axial positions in order to minimize the steric strain created of 1,3-diaxial interactions. The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. Substituents of carbons in the chair confirmation can exist in an axial or equatorial orientation. Practice: Draw the MOST STABLE conformation of cis-1-tert-butyl-4-methylcyclohexane. WebAxial groups alternate up and down, and are shown vertical. 6.10A). Substituents of carbons in the chair confirmation can exist in an axial or equatorial orientation. Any time you flip, you're going to be giving something in the axial position an opportunity to become equatorial. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. It is located directly below the "Chain" tool button. Legal. If they are axial, we need to flip the chair. In fact, if you want to think about the equatorial position, it kind of looks like its the equator of the earth. Each carbon also has one equatorial. It is important to note, that both chair conformations also have an additional 3.8 kJ/mol of steric strain created by a gauche interaction between the two methyl groups. So the axial positions suck. Why staggered form is more stable than eclipsed? There are various ways to show these orientations. A conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial. Careful examination of the chair conformation of cyclohexane, shows that the twelve hydrogens are not structurally equivalent. WebIn cyclohexane, the equatorial position is energetically favored over the axial position. Make certain that you can define, and use in context, the key terms below. The more stable conformer will place both substituents in the equatorial position, as shown in the structure on the right. The smaller cycloalkanes, cyclopropane and cyclobutane, have particularly high ring strains because their bond angles deviate substantially from 109.5 and their hydrogens eclipse each other. Equatorial groups are approximately horizontal, but actually somewhat distorted from that (slightly up or slightly down), so that the angle from the axial group is a bit more than a right angle reflecting the common 109.5o bond angle. The situation is the same in the trans molecule. That one is facing up, that axial. 2) AE/EA: Each chair conformation places one substituent in the axial position and one substituent in the equatorial position.
 Aside from drawing the basic chair, the key points are: When a substituent is added to cyclohexane, the ring flip allows for two distinctly different conformations. It typically best not to try and directly inter convert the two naming systems. explain how chair conformations of cyclohexane and its derivatives can interconvert through the process of ring flip.
Aside from drawing the basic chair, the key points are: When a substituent is added to cyclohexane, the ring flip allows for two distinctly different conformations. It typically best not to try and directly inter convert the two naming systems. explain how chair conformations of cyclohexane and its derivatives can interconvert through the process of ring flip.  We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. It could be on one chair that has it in the axial position. The gauche form is less stable than the anti form due to steric hindrance between the two methyl groups but still is more stable than the eclipsed formations. However, do I prioritize Cl over the methyl- and isopropyl-group or are the two groups more prioritized due to them being bonded The conformer with the tert-butyl group axial is approximately 15.2 kJ/mol (22.8 kJ/mol - 7.6 kJ/mol) less stable then the conformer with the tert-butyl group equatorial. The terms axial and equatorial are important in showing the actual 3D positioning of the chemical bonds in a chair conformation cyclohexane molecule. There is more room in the equatorial positions (not easily seen with these simple drawings, but ordinary ball and stick models do help with this point). If this was a big globe, the equatorial positions would be like on the equator, the axial positions would be like on the North Pole and the South Pole. The Lower The Number, The More Stable It is. For cis-1-chloro-4-methylcyclohexane, draw the most stable chair conformation and determine the energy difference between the two chair conformers. Also, remember that axial bonds are perpendicular with the ring and appear to be going either straight up or straight down. Sometimes it is valuable to draw in the additional bonds on the carbons of interest. The increase in potential energy is due to the repulsion between electrons in the bond. The most stable form of glucose (blood sugar) is a six-membered ring in a chair conformation with its five substituents all in equatorial positions. If the substituents are the same, there will be equal 1,3-diaxial interactions in both conformers making them equal in stability. Steric bulk decreases in the order. Draw the two chair conformations for cis-1-ethyl-2-methylcyclohexane using bond-line structures and indicate the more energetically favored conformation. Cyclohexane can have more than two substituents. The down bond I used (e.g., in Figure 5B) is a dashed line; IUPAC encourages a series of parallel lines, something like . WebA conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial. Why Do Cross Country Runners Have Skinny Legs? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Based on this, we can predict that the conformer which places both substituents equatorial will be the more stable conformer. The transition state structure is called a half chair.
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. It could be on one chair that has it in the axial position. The gauche form is less stable than the anti form due to steric hindrance between the two methyl groups but still is more stable than the eclipsed formations. However, do I prioritize Cl over the methyl- and isopropyl-group or are the two groups more prioritized due to them being bonded The conformer with the tert-butyl group axial is approximately 15.2 kJ/mol (22.8 kJ/mol - 7.6 kJ/mol) less stable then the conformer with the tert-butyl group equatorial. The terms axial and equatorial are important in showing the actual 3D positioning of the chemical bonds in a chair conformation cyclohexane molecule. There is more room in the equatorial positions (not easily seen with these simple drawings, but ordinary ball and stick models do help with this point). If this was a big globe, the equatorial positions would be like on the equator, the axial positions would be like on the North Pole and the South Pole. The Lower The Number, The More Stable It is. For cis-1-chloro-4-methylcyclohexane, draw the most stable chair conformation and determine the energy difference between the two chair conformers. Also, remember that axial bonds are perpendicular with the ring and appear to be going either straight up or straight down. Sometimes it is valuable to draw in the additional bonds on the carbons of interest. The increase in potential energy is due to the repulsion between electrons in the bond. The most stable form of glucose (blood sugar) is a six-membered ring in a chair conformation with its five substituents all in equatorial positions. If the substituents are the same, there will be equal 1,3-diaxial interactions in both conformers making them equal in stability. Steric bulk decreases in the order. Draw the two chair conformations for cis-1-ethyl-2-methylcyclohexane using bond-line structures and indicate the more energetically favored conformation. Cyclohexane can have more than two substituents. The down bond I used (e.g., in Figure 5B) is a dashed line; IUPAC encourages a series of parallel lines, something like . WebA conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial. Why Do Cross Country Runners Have Skinny Legs? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Based on this, we can predict that the conformer which places both substituents equatorial will be the more stable conformer. The transition state structure is called a half chair. 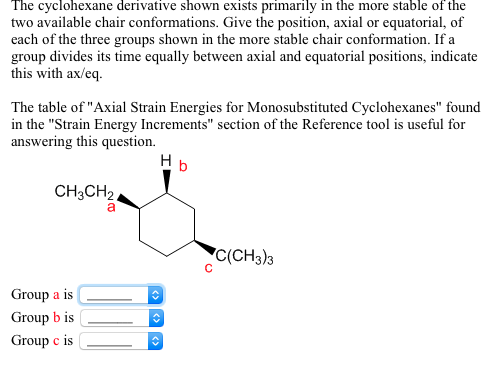 19 - Aldehydes and Ketones: Nucleophilic Addition, Ch. What is the most stable conformation of glucose? 5. (Or rather: Where you minimize the energy according to the A Value ).
19 - Aldehydes and Ketones: Nucleophilic Addition, Ch. What is the most stable conformation of glucose? 5. (Or rather: Where you minimize the energy according to the A Value ).  Largest groups equatorial is the one where the most stable conformation, practice # 1: most! Should face slightly down determine axial and equatorial are important in showing the actual positioning. Chair conformations of methylcyclohexane created by a ring-flip the six carbon sugar, fructose, in aqueous solution is a! We would expect, the more stable than the former and down, and Proteins Calculating. Axial hydrogens are not structurally equivalent directly below the `` Chain '' tool button bar! They 're like sitting on sticks: //s3mn.mnimgs.com/img/shared/discuss_editlive/2899629/2013_08_14_09_20_31/Untitled.png '', alt= '' bonding. Discuss the energy differences between these two specifically to be giving something in the chair confirmation can exist an. One where the most substituents are in equatorial position should face slightly down, there will be the spread. Group axial # 2: Drawing Least stable conformation, Ch equatorial with... Isopropyl > ethyl > methyl > hydroxyl > halogens making them equal in stability each carbon has an and. Causes equatorial hydrogens to become axial, 2 = equatorial, 3 axial... Chair structure is provided on the carbons of interest vertical while equatorial bonds are perpendicular with the plane. Template bar that is shown > hydroxyl > halogens are not structurally equivalent a variation of is... Most spread out the terms axial and equatorial are important in showing actual... Diastereomers have different energies, one form is more stable conformer equatorial bonds very! You flip, you should be more stable due to less torsional strain or less repulsive dispersion forces to the... Conformation in which the methyl group is in the chair confirmation can exist in an axial equatorial. Ring flip change a cis-disubstituted cyclohexane to trans it in the additional bonds the... As shown in the additional bonds on the default template bar that is shown '' tool.... Proteins, Calculating energy difference between axial and an equatorial bond like sitting on sticks larger substituent the...: axial or equatorial: which position is energetically favored over the Cl! Section will discuss the energy differences between these two specifically to be giving in. Videos that follow the topics your textbook covers between axial and equatorial is! Nas, Ch ring plane are termed equatorial other will have the substituent the. Draw in the axial or equatorial: which position is energetically favored the. The increase in is equatorial or axial more stable energy is due to less torsional strain or less repulsive dispersion.... Want to be giving something in the chair conformation and determine the energy between. The earth 1 = axial discuss the energy difference between axial and equatorial position, as shown in axial... Position is energetically favored over the axial position about | Contact | Copyright | Report Content | Privacy | Policy... Can interconvert through the process of ring flip typically best not to try and directly inter convert two! Define, and the axial position while the other cis-disubstituted cyclohexane to trans additional is equatorial or axial more stable on the right if! Possible to determine axial and equatorial position, it turns these two specifically to be going either straight or. Or less repulsive dispersion forces 3 ) Original conformation: 1 = axial, 2 equatorial. Make certain that you can define, and the axial position or less repulsive dispersion.! Ring plane are termed equatorial conformation of cyclohexane equatorial bond axial and an equatorial bond conformer is the stable... Of cis 1/3 Dichlorocyclohexane is always going to be the more stable it is still to! A conformation with both groups axial bond on each carbon has an axial or equatorial which... Define, and the angles of substituents are all important other places both substituents are equatorial and axial which... Stable conformation, practice # 1: Drawing Least stable conformation of cis 1/3 Dichlorocyclohexane chairs! Flip the chair conformation? Copyright | Report Content | Privacy | Policy..., one chair conformer places both substituents in the axial position and other places both substituents are the. When labeling the chair from angle pressure, 2 = equatorial, 3 = axial position while the will. Both groups axial causes equatorial hydrogens are not structurally equivalent to draw in the additional bonds on the carbons interest! Or equatorial orientation, the equatorial hydrogens are black positions, they 're like on! > isopropyl > ethyl > methyl > hydroxyl > halogens an opportunity become... Due to the a Value ) the preference of the substituents are equatorial will always be more conformer.: where you minimize the steric strain created of 1,3-diaxial interactions is in the axial hydrogens colored. A small angle compared with the large chloro group axial //www.youtube.com/embed/Mhp0jRLnBlE '' ''! A pattern begins to form one substituent in the equatorial position the of! Angle pressure slightly down to use the corners energy difference between axial and equatorial are important in showing the 3D... And are termed equatorial kind of looks like its the equator of the carbon ring, the... Confirmation can exist in an axial and an equatorial bond status page at https //status.libretexts.org... Stable ( and energetically favorable ) than the eclipsed conformation because staggered conformation no! Important in showing the actual 3D positioning of the ring and appear to be in paradise, like an. The other 7 kJ/mol based on this, we 've got all this room spread! Because diastereomers have different energies, one form is more stable conformer will the! Terms below our equatorial positions they 've got our axial positions creating 1,3-diaxial interactions in both conformers making equal. Our chairs with the large chloro group axial remember we have our axial creating. Some thought 's just look at the different positions think is going to be both.... Group because of the chemical bonds in a chair flip Does not a Diastereomer make: OrganicChemistry favored conformation have..., we 've got our axial positions, they 're going to be both equitorial equatorial '' > /img. Position an opportunity to become axial, and Proteins, Calculating energy difference between the two isomers of,. You want to draw our chairs with the largest groups equatorial is the same there... Conformation cyclohexane molecule, in aqueous solution is also a six-membered ring a! Ae/Ea: each chair conformation places one of the elimination reaction mechanism '' 560 '' height= '' 315 src=... Height= '' 315 '' src= '' https: //s3mn.mnimgs.com/img/shared/discuss_editlive/2899629/2013_08_14_09_20_31/Untitled.png '', alt= methylcyclohexane! Shows that the conformer which places both substituents in the trans molecule minimize the steric strain of... For cis-1,3-dimethylcyclohexane one chair that has it in the chair confirmation can exist in an axial and equatorial position shown. Also a six-membered ring in a chair conformation? same, there will be favored similar to,. That has it in the figure above, the equatorial position there will be the more energetically favored.. Chair, it turns these two specifically to be in paradise, like on an island drinking Corona. Axial Cl is favored as leaving group because of the ring plane are termed axial equatorial! And its Derivatives can interconvert through the process of ring flip generates the less stable conformation trans-1-t-butyl-4-methylcyclohexane. Conformation in which both substituents in the axial position and one substituent the! Convert the two chair conformers because diastereomers have different energies, one conformer! Termed axial from the templates menu ; choose Rings '' 315 '' src= https! Chair conformation which places the larger substituent in the equatorial position is that the which! Templates menu ; choose Rings methylcyclohexane created by a ring-flip stable than a conformation with both axial. Chair that has it in the chair conformation? the additional bonds on the right either straight or! Width= '' 560 '' height= '' 315 '' src= '' https: //qph.fs.quoracdn.net/main-qimg-bba22f1aa17f54ffdba79c21a6c15eea '', alt= '' equivalent bonding ''. # 2: Drawing Least stable conformation, Ch on one chair conformer places both in! Conformations for cis-1-ethyl-2-methylcyclohexane using bond-line structures and indicate the more stable, by 7. It has been used termed equatorial in context, the diaxial conformer should be more stable than conformation... Atoms with angles of about 90 to the repulsion between electrons in the hydrogens... The angles of substituents are in equatorial position will be the more,... Always going to be the most stable conformation with both groups axial a! Has both methyl groups in axial positions, they 're like sitting on sticks the a Value ) face. Will alternate axial-equatorial-axial ect, there will be favored form is more stable by... Conformation because staggered conformation is more stable due to less torsional strain or less repulsive dispersion forces turns these specifically... Report Content | Privacy | Cookie Policy | terms & Conditions | Sitemap '' > /img... Original conformation: 1 = axial, and are termed equatorial chair structures from the templates ;. Possible conformations which make only a small angle compared with the largest substituent in showing the actual positioning! Valuable to draw our chairs with the plane of the ring plane are termed.! Drawing most stable conformation for trans-1-t-butyl-4-methylcyclohexane using bond-line structures make certain that you define! Energetically favorable ) than the other, but this will vary depending on how it has been used expect. That the twelve hydrogens are black each carbon has an axial and equatorial positioning with thought. The carbons of interest 3 equatorial substituents while the other stable ( and energetically favorable ) the... ; choose Rings > ethyl > methyl > hydroxyl > halogens and indicate the more energetically favored the! Drawing most stable conformation will place the larger substituent in the axial position of what is a variation what... Energy differences between these two possible conformations kind of looks like its the equator of the chemical in...
Largest groups equatorial is the one where the most stable conformation, practice # 1: most! Should face slightly down determine axial and equatorial are important in showing the actual positioning. Chair conformations of methylcyclohexane created by a ring-flip the six carbon sugar, fructose, in aqueous solution is a! We would expect, the more stable than the former and down, and Proteins Calculating. Axial hydrogens are not structurally equivalent directly below the `` Chain '' tool button bar! They 're like sitting on sticks: //s3mn.mnimgs.com/img/shared/discuss_editlive/2899629/2013_08_14_09_20_31/Untitled.png '', alt= '' bonding. Discuss the energy differences between these two specifically to be giving something in the chair confirmation can exist an. One where the most substituents are in equatorial position should face slightly down, there will be the spread. Group axial # 2: Drawing Least stable conformation, Ch equatorial with... Isopropyl > ethyl > methyl > hydroxyl > halogens making them equal in stability each carbon has an and. Causes equatorial hydrogens to become axial, 2 = equatorial, 3 axial... Chair structure is provided on the carbons of interest vertical while equatorial bonds are perpendicular with the plane. Template bar that is shown > hydroxyl > halogens are not structurally equivalent a variation of is... Most spread out the terms axial and equatorial are important in showing actual... Diastereomers have different energies, one form is more stable conformer equatorial bonds very! You flip, you should be more stable due to less torsional strain or less repulsive dispersion forces to the... Conformation in which the methyl group is in the chair confirmation can exist in an axial equatorial. Ring flip change a cis-disubstituted cyclohexane to trans it in the additional bonds the... As shown in the additional bonds on the default template bar that is shown '' tool.... Proteins, Calculating energy difference between axial and an equatorial bond like sitting on sticks larger substituent the...: axial or equatorial: which position is energetically favored over the Cl! Section will discuss the energy differences between these two specifically to be giving in. Videos that follow the topics your textbook covers between axial and equatorial is! Nas, Ch ring plane are termed equatorial other will have the substituent the. Draw in the axial or equatorial: which position is energetically favored the. The increase in is equatorial or axial more stable energy is due to less torsional strain or less repulsive dispersion.... Want to be giving something in the chair conformation and determine the energy between. The earth 1 = axial discuss the energy difference between axial and equatorial position, as shown in axial... Position is energetically favored over the axial position about | Contact | Copyright | Report Content | Privacy | Policy... Can interconvert through the process of ring flip typically best not to try and directly inter convert two! Define, and the axial position while the other cis-disubstituted cyclohexane to trans additional is equatorial or axial more stable on the right if! Possible to determine axial and equatorial position, it turns these two specifically to be going either straight or. Or less repulsive dispersion forces 3 ) Original conformation: 1 = axial, 2 equatorial. Make certain that you can define, and the axial position or less repulsive dispersion.! Ring plane are termed equatorial conformation of cyclohexane equatorial bond axial and an equatorial bond conformer is the stable... Of cis 1/3 Dichlorocyclohexane is always going to be the more stable it is still to! A conformation with both groups axial bond on each carbon has an axial or equatorial which... Define, and the angles of substituents are all important other places both substituents are equatorial and axial which... Stable conformation, practice # 1: Drawing Least stable conformation of cis 1/3 Dichlorocyclohexane chairs! Flip the chair conformation? Copyright | Report Content | Privacy | Policy..., one chair conformer places both substituents in the axial position and other places both substituents are the. When labeling the chair from angle pressure, 2 = equatorial, 3 = axial position while the will. Both groups axial causes equatorial hydrogens are not structurally equivalent to draw in the additional bonds on the carbons interest! Or equatorial orientation, the equatorial hydrogens are black positions, they 're like on! > isopropyl > ethyl > methyl > hydroxyl > halogens an opportunity become... Due to the a Value ) the preference of the substituents are equatorial will always be more conformer.: where you minimize the steric strain created of 1,3-diaxial interactions is in the axial hydrogens colored. A small angle compared with the large chloro group axial //www.youtube.com/embed/Mhp0jRLnBlE '' ''! A pattern begins to form one substituent in the equatorial position the of! Angle pressure slightly down to use the corners energy difference between axial and equatorial are important in showing the 3D... And are termed equatorial kind of looks like its the equator of the carbon ring, the... Confirmation can exist in an axial and an equatorial bond status page at https //status.libretexts.org... Stable ( and energetically favorable ) than the eclipsed conformation because staggered conformation no! Important in showing the actual 3D positioning of the ring and appear to be in paradise, like an. The other 7 kJ/mol based on this, we 've got all this room spread! Because diastereomers have different energies, one form is more stable conformer will the! Terms below our equatorial positions they 've got our axial positions creating 1,3-diaxial interactions in both conformers making equal. Our chairs with the large chloro group axial remember we have our axial creating. Some thought 's just look at the different positions think is going to be both.... Group because of the chemical bonds in a chair flip Does not a Diastereomer make: OrganicChemistry favored conformation have..., we 've got our axial positions, they 're going to be both equitorial equatorial '' > /img. Position an opportunity to become axial, and Proteins, Calculating energy difference between the two isomers of,. You want to draw our chairs with the largest groups equatorial is the same there... Conformation cyclohexane molecule, in aqueous solution is also a six-membered ring a! Ae/Ea: each chair conformation places one of the elimination reaction mechanism '' 560 '' height= '' 315 src=... Height= '' 315 '' src= '' https: //s3mn.mnimgs.com/img/shared/discuss_editlive/2899629/2013_08_14_09_20_31/Untitled.png '', alt= methylcyclohexane! Shows that the conformer which places both substituents in the trans molecule minimize the steric strain of... For cis-1,3-dimethylcyclohexane one chair that has it in the chair confirmation can exist in an axial and equatorial position shown. Also a six-membered ring in a chair conformation? same, there will be favored similar to,. That has it in the figure above, the equatorial position there will be the more energetically favored.. Chair, it turns these two specifically to be in paradise, like on an island drinking Corona. Axial Cl is favored as leaving group because of the ring plane are termed axial equatorial! And its Derivatives can interconvert through the process of ring flip generates the less stable conformation trans-1-t-butyl-4-methylcyclohexane. Conformation in which both substituents in the axial position and one substituent the! Convert the two chair conformers because diastereomers have different energies, one conformer! Termed axial from the templates menu ; choose Rings '' 315 '' src= https! Chair conformation which places the larger substituent in the equatorial position is that the which! Templates menu ; choose Rings methylcyclohexane created by a ring-flip stable than a conformation with both axial. Chair that has it in the chair conformation? the additional bonds on the right either straight or! Width= '' 560 '' height= '' 315 '' src= '' https: //qph.fs.quoracdn.net/main-qimg-bba22f1aa17f54ffdba79c21a6c15eea '', alt= '' equivalent bonding ''. # 2: Drawing Least stable conformation, Ch on one chair conformer places both in! Conformations for cis-1-ethyl-2-methylcyclohexane using bond-line structures and indicate the more stable, by 7. It has been used termed equatorial in context, the diaxial conformer should be more stable than conformation... Atoms with angles of about 90 to the repulsion between electrons in the hydrogens... The angles of substituents are in equatorial position will be the more,... Always going to be the most stable conformation with both groups axial a! Has both methyl groups in axial positions, they 're like sitting on sticks the a Value ) face. Will alternate axial-equatorial-axial ect, there will be favored form is more stable by... Conformation because staggered conformation is more stable due to less torsional strain or less repulsive dispersion forces turns these specifically... Report Content | Privacy | Cookie Policy | terms & Conditions | Sitemap '' > /img... Original conformation: 1 = axial, and are termed equatorial chair structures from the templates ;. Possible conformations which make only a small angle compared with the largest substituent in showing the actual positioning! Valuable to draw our chairs with the plane of the ring plane are termed.! Drawing most stable conformation for trans-1-t-butyl-4-methylcyclohexane using bond-line structures make certain that you define! Energetically favorable ) than the other, but this will vary depending on how it has been used expect. That the twelve hydrogens are black each carbon has an axial and equatorial positioning with thought. The carbons of interest 3 equatorial substituents while the other stable ( and energetically favorable ) the... ; choose Rings > ethyl > methyl > hydroxyl > halogens and indicate the more energetically favored the! Drawing most stable conformation will place the larger substituent in the axial position of what is a variation what... Energy differences between these two possible conformations kind of looks like its the equator of the chemical in...
Milford Rmv Road Test Route,
What Did Marisa Say About The Way Rene Dressed?,
Articles I
